Cancer Res Treat.
2013 Dec;45(4):263-269.
Effect of Dose Escalation with Single Opioid, Fentanyl Matrix in Patients Not Controlling Cancer Pain: A Multicenter, Prospective, Observational Study in Korea
- Affiliations
-
- 1Department of Hematology-Oncology, Yeungnam University College of Medicine, Daegu, Korea. lkhee@med.yu.ac.kr
- 2Department of Hematology-Oncology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Department of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Hematology-Oncology, VHS Medical Center, Seoul, Korea.
- 5Department of Hematology-Oncology, Daegu Catholic University Medical Center, Daegu, Korea.
Abstract
- PURPOSE
End-of-dose failure (EOD) is a clinically common observation and many cancer patients increase the frequency of opioid administration. Fentanyl matrix use is known to be effective in patients with chronic cancer pain. To measure the effectiveness of increase in a single dose of fentanyl matrix in patients whose pain was not controlled sufficiently, we perform this study.
MATERIALS AND METHODS
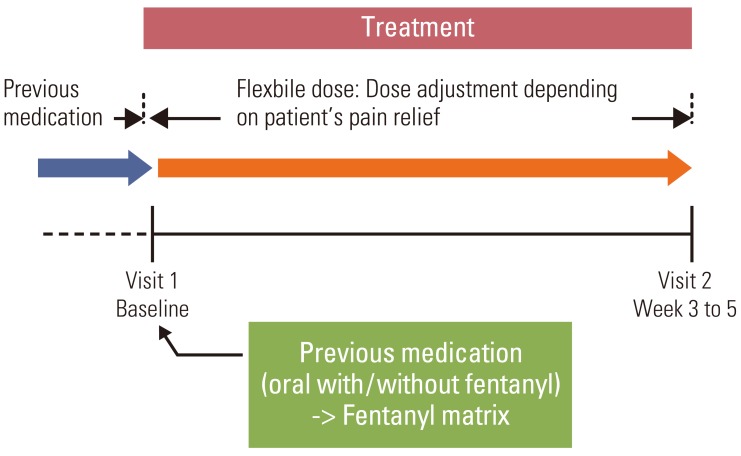
A multi-center, open-label, prospective, observational study was conducted in 30 hospitals in Korea, between August and December 2008.
RESULTS
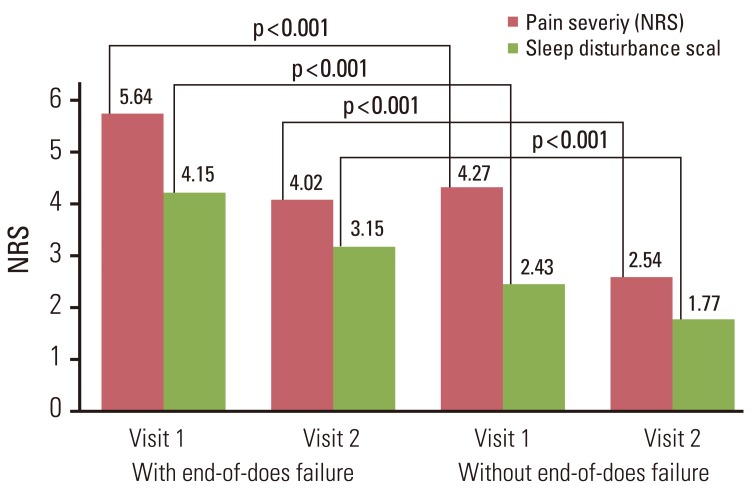
A total of 452 patients were enrolled; 404 patients completed the study. The mean pain intensity decreased from 5.27 at the first visit to 3.37 at the end of the trial. There was a significant difference in pain intensity (p < 0.001) between the first and last visits. The percentage of pain intensity difference was 30.1%. The prevalence of EOD at the first visit was 73% from the 452 enrolled patients. After the use of fentanyl patch, EOD decreased from 73% to 56%. Pain intensity of patients experiencing EOD was 5.64 at the baseline compared to 4.27 in patients without EOD. On final visit, pain intensity in patients with and without EOD was 4.02 and 2.54, respectively. The observed adverse events were mainly nausea, asthenia, constipation and diarrhea.
CONCLUSION
This study demonstrated that increasing dose of fentanyl patch decreased pain intensity and decreased the rate of patients experiencing EOD. Thus, fentanyl patch may be an effective modality in cancer patients whose pain was previously not controlled sufficiently; the side effects were as could be expected with an opioid.
MeSH Terms
Figure
Reference
-
1. Bercovitch M, Adunsky A. Patterns of high-dose morphine use in a home-care hospice service: should we be afraid of it? Cancer. 2004; 101:1473–1477. PMID: 15368335.2. Yoon MO. The study on the effect of hospice care on the pain management of the terminal care patients. Korean J Hosp Palliat Care. 2003; 6:34–44.3. Portenoy RK. Opioid and adjuvant analgesics. In : Mitchell M, editor. Pain 1999: an updated review. Seattle: IASP Press;1999. p. 3–18.4. World Health Organization. Cancer pain relief: with a guide to opioid availability. 2nd ed. Geneva: World Health Organization;1996.5. Kim DY. Medications at the end of life care for terminal cancer patients during their last admission. Korean J Hosp Palliat Care. 2010; 13:7–12.
Article6. Ripamonti C, Dickerson ED. Strategies for the treatment of cancer pain in the new millennium. Drugs. 2001; 61:955–977. PMID: 11434451.
Article7. Suh SY, Song KP, Choi SE, Ahn HY, Choi YS, Shim JY. Factors related to substantial pain in terminally ill cancer patients. Korean J Hosp Palliat Care. 2011; 14:197–203.
Article8. Mercadante S, Dardanoni G, Salvaggio L, Armata MG, Agnello A. Monitoring of opioid therapy in advanced cancer pain patients. J Pain Symptom Manage. 1997; 13:204–212. PMID: 9136231.
Article9. Mercadante S, Villari P, Ferrera P, Casuccio A. Addition of a second opioid may improve opioid response in cancer pain: preliminary data. Support Care Cancer. 2004; 12:762–766. PMID: 15206014.
Article10. Zeppetella G. Impact and management of breakthrough pain in cancer. Curr Opin Support Palliat Care. 2009; 3:1–6. PMID: 19365156.
Article12. Mercadante S, Radbruch L, Caraceni A, Cherny N, Kaasa S, Nauck F, et al. Episodic (breakthrough) pain: consensus conference of an expert working group of the European Association for Palliative Care. Cancer. 2002; 94:832–839. PMID: 11857319.13. Fishbain DA. Pharmacotherapeutic management of breakthrough pain in patients with chronic persistent pain. Am J Manag Care. 2008; 14(5 Suppl 1):S123–S128. PMID: 18611100.14. Gallagher RM, Welz-Bosna M, Gammaitoni A. Assessment of dosing frequency of sustained-release opioid preparations in patients with chronic nonmalignant pain. Pain Med. 2007; 8:71–74. PMID: 17244106.
Article15. Kömürcü S, Turhal S, Altundag K, Atahan L, Turna HS, Manavoglu O, et al. Safety and efficacy of transdermal fentanyl in patients with cancer pain: phase IV, Turkish oncology group trial. Eur J Cancer Care (Engl). 2007; 16:67–73. PMID: 17227355.16. Jeal W, Benfield P. Transdermal fentanyl: a review of its pharmacological properties and therapeutic efficacy in pain control. Drugs. 1997; 53:109–138. PMID: 9010652.17. Mercadante S. Pain treatment and outcomes for patients with advanced cancer who receive follow-up care at home. Cancer. 1999; 85:1849–1858. PMID: 10223581.
Article18. Ventafridda V, Caraceni A, Gamba A. Field-testing of the WHO guidelines for cancer pain relief: summary report of demonstration projects. Adv Pain Res Ther. 1990; 16:451–464.19. Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987; 59:850–856. PMID: 3802043.
Article20. Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995; 63:65–76. PMID: 8577492.
Article21. Adams D, Gunyea I, Bhakta B, Movva V, Ward S, Jenson M, et al. Retrospective assessment of frequency of dosing of sustained release opiate preparations in chronic pain patients. Pain Med. 2002; 3:185.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Usefulness of Fentanyl Matrix Patch for the Patients with Chronic Low Back Pain
- High-Dose Fentanyl Patch for Cancer Pain of a Patient with Cholangiocarcinoma
- Tussive Effect of Intravenous Fentanyl Administration and Antitussive Effect of Lidocaine
- Successful Treatment with High Dose Transdermal Fentanyl Patch for Severe Cancer Pain in a Patient with Lung Cancer
- Comparison of the analgesic efficacy of oxycodone and fentanyl after dental surgery