Ann Surg Treat Res.
2015 May;88(5):276-280. 10.4174/astr.2015.88.5.276.
Noninvasive monitoring of mouse renal allograft rejection using micro-CT
- Affiliations
-
- 1Department of Urology, Huashan Hospital, Fudan University, Shanghai, China.
- 2Division of Transplantation Immunology, National Research Institute for Child Health and Development, Tokyo, Japan. ri-k@ncchd.go.jp
- 3AIDS Research Center, National Institute of Infectious Diseases, Tokyo, Japan.
- KMID: 2166996
- DOI: http://doi.org/10.4174/astr.2015.88.5.276
Abstract
- PURPOSE
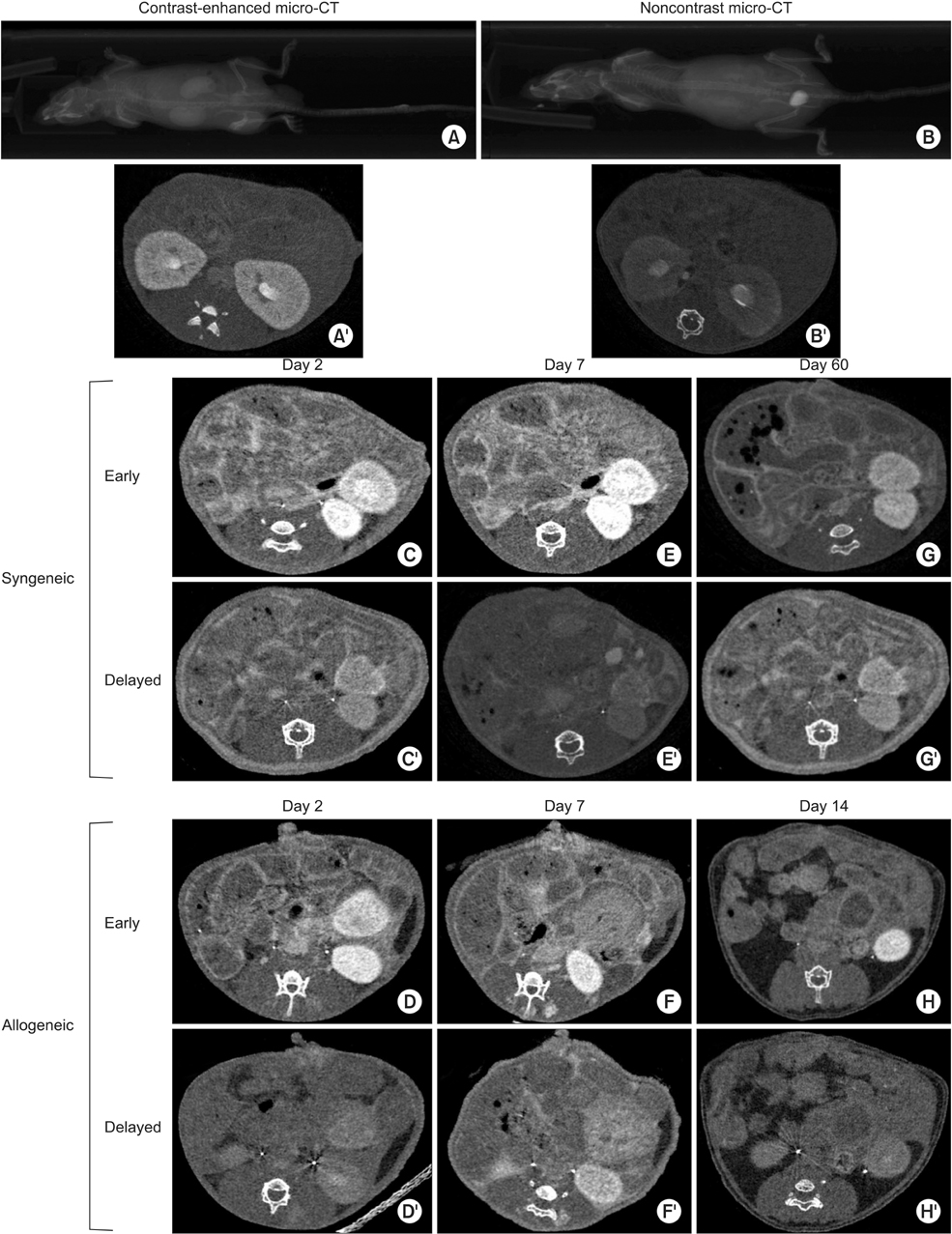
Acute renal graft rejection can only be definitively diagnosed by renal biopsy. However, biopsies carry a risk of renal transplant injury and loss. Micro-CT is widely used in preclinical studies of small animals. Here, we propose micro-CT could noninvasively monitor and evaluate renal location and function in a mouse kidney transplant model.
METHODS
Orthotopic kidney transplantation was performed in a BALB/c -to- C57BL/6j or C57BL/6j-to- C57BL/6j mouse model. After optimizing imaging techniques, five mice were imaged with micro-CT and the findings were verified histologically.
RESULTS
Micro-CT can monitor and evaluate renal location and function after orthotopic kidney transplantation. There were no mice deaths while renal transplants were failure.
CONCLUSION
We propose that graft micro-CT imaging is a new option that is noninvasive and specific, and can aid in early detection and follow-up of acute renal rejection. This method is potentially useful to improve posttransplant rejection monitoring.
Keyword
MeSH Terms
Figure
Reference
-
1. Ge F, Gong W. Strategies for successfully establishing a kidney transplant in a mouse model. Exp Clin Transplant. 2011; 9:287–294.2. Tse GH, Hughes J, Marson LP. Systematic review of mouse kidney transplantation. Transpl Int. 2013; 26:1149–1160.3. Schambach SJ, Bag S, Schilling L, Groden C, Brockmann MA. Application of micro-CT in small animal imaging. Methods. 2010; 50:2–13.4. Azuma H, Isaka Y, Nomi H, Inamoto T, Li XK, Hounig T, et al. Induction of donorspecific tolerance using superagonistic CD28 antibody in rat renal allografts: regulatory T-cell expansion before engraftment may be important. Transplantation. 2010; 90:1328–1335.5. Abe T, Li XK, Yazawa K, Hatayama N, Xie L, Sato B, et al. Hydrogen-rich University of Wisconsin solution attenuates renal cold ischemia-reperfusion injury. Transplantation. 2012; 94:14–21.6. Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008; 7:591–607.7. Soltysiak P, Saxena AK. Micro-computed tomography for implantation site imaging during in situ oesophagus tissue engineering in a live small animal model. J Tissue Eng Regen Med. 2009; 3:573–576.8. Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Masyuk AI, Gradilone SA, et al. Inhibition of Cdc25A suppresses hepato-renal cystogenesis in rodent models of polycystic kidney and liver disease. Gastroenterology. 2012; 142:622–633.e4.9. Vierling JM, Hreha G, Wang H, Braun M. The role of biliary epithelial cells in the immunopathogenesis of non-suppurative destructive cholangitis in murine hepatic graft-versus-host disease. Trans Am Clin Climatol Assoc. 2011; 122:326–335.10. Paulus MJ, Gleason SS, Kennel SJ, Hunsicker PR, Johnson DK. High resolution X-ray computed tomography: an emerging tool for small animal cancer research. Neoplasia. 2000; 2:62–70.11. Marx J. Imaging. Animal models: live and in color. Science. 2003; 302:1880–1882.12. Maehara N. Experimental microcomputed tomography study of the 3D microangioarchitecture of tumors. Eur Radiol. 2003; 13:1559–1565.13. Cai QY, Lee H, Kim EJ, Moon H, Chang K, Rho J, et al. Magnetic resonance imaging of superparamagnetic iron oxide-labeled macrophage infiltrates in acute-phase renal ischemia-reperfusion mouse model. Nanomedicine. 2012; 8:365–373.14. Reuter S, Schnockel U, Schroter R, Schober O, Pavenstadt H, Schafers M, et al. Noninvasive imaging of acute renal allograft rejection in rats using small animal FFDG-PET. PLoS One. 2009; 4:e5296.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunologic monitoring in kidney transplant recipients
- Diagnosis of renal transplant rejection: Banff classification and beyond
- Impact of the Pattern of Acute Rejection Episodes on Graft Survival
- Allograft Immune Reaction of Kidney Transplantation: Part 1. Mechanism of Allograft Rejection
- A Case of Spontaneous Rupture of REnal Allograft