Healthc Inform Res.
2016 Jan;22(1):39-45. 10.4258/hir.2016.22.1.39.
New Alert Override Codes for the Drug Utilization Review System Derived from Outpatient Prescription Data from a Tertiary Teaching Hospital in Korea
- Affiliations
-
- 1Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea. jcvara@hanmil.net
- 2Department of Healthcare Management, Eulji University, Seongnam, Korea.
- 3Department of Public Health and Medical Administration, Dongyang University, Yeongju, Korea.
- 4Mibyeong Research Center, Korea Institute of Oriental Medicine, Daejeon, Korea.
- 5Department of Nursing, Dongyang University, Yeongju, Korea.
- KMID: 2166915
- DOI: http://doi.org/10.4258/hir.2016.22.1.39
Abstract
OBJECTIVES
This paper proposes new alert override reason codes that are improvements on existing Drug Utilization Review (DUR) codes based on an analysis of DUR alert override cases in a tertiary medical institution.
METHODS
Data were obtained from a tertiary teaching hospital covering the period from April 1, 2012 to January 15, 2013. We analyzed cases in which doctors had used the 11 overlapping prescription codes provided by the Health Insurance Review and Assessment Service (HIRA) or had provided free-text reasons.
RESULTS
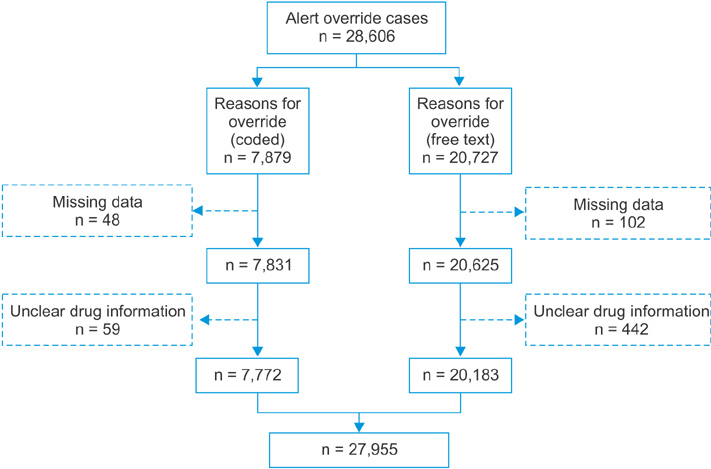
We identified 27,955 alert override cases. Among these, 7,772 (27.8%) utilized the HIRA codes, and 20,183 (72.2%) utilized free-text reasons. According to the free-text content analysis, 8,646 cases (42.8%) could be classified using the 11 HIRA codes, and 11,537 (57.2%) could not. In the unclassifiable cases, we identified the need for codes for "prescription relating to operation" and "emergency situations." Two overlapping prescription codes required removal because they were not used. Codes A, C, F, H, I, and J (for drug non-administration cases) explained surrounding situations in too much detail, making differentiation between them difficult. These 6 codes were merged into code J4: "patient was not taking/will not take the medications involved in the DDI." Of the 11 HIRA codes, 6 were merged into a single code, 2 were removed, and 2 were added, yielding 6 alert override codes. We could codify 23,550 (84.2%) alert override cases using these codes.
CONCLUSIONS
These new codes will facilitate the use of the drug-drug interactions alert override in the current DUR system. For further study, an appropriate evaluation should be conducted with prescribing clinicians.
Keyword
MeSH Terms
Figure
Reference
-
1. Choi NK, Park BJ. Strategy for establishing an effective Korean drug utilization review system. J Korean Med Assoc. 2010; 53(12):1130–1138.
Article2. Choi JS, Kim DS. A case study of implementation of concurrent drug utilization review system at a general hospital. J Korean Inst Ind Eng. 2013; 39(1):20–29.
Article3. Strom BL, Kimmel SE, Hennessy S. Pharmacoepidemiology. 5th ed. Chichester: Wiley-Blackwell;2012.4. Jeon HL, Park JH, Kim DS, Choi BC. Review of the Drug Utilization Review program of United States 47 States and suggestion of policy. Health Soc Welf Rev. 2014; 34(3):192–221.
Article5. Kim DS, Park JH, Jeon HL, Park CM, Kang HA. The effect of Korean Prospective Drug Utilization Review Program on the prescription rate of drug-drug interactions. Health Policy Manag. 2014; 24(2):120–127.
Article6. Brunton L, Chabner B, Knollmann B. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York (NY): McGraw-Hill;2011.7. Rodrigues AD. Drug-drug interactions. 2nd ed. New York (NY): Informa Healthcare;2008.8. Hines LE, Murphy JE. Potentially harmful drug-drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011; 9(6):364–377.
Article9. Hansten PD, Horn JR. Drug interactions analysis and management. 6th ed. St. Louis (MO): Wolters Kluwers Health;2011.10. Grizzle AJ, Mahmood MH, Ko Y, Murphy JE, Armstrong EP, Skrepnek GH, et al. Reasons provided by prescribers when overriding drug-drug interaction alerts. Am J Manag Care. 2007; 13:573–578.11. Ahn EK, Cho SY, Shin D, Jang C, Park RW. Differences of reasons for alert overrides on contraindicated co-prescriptions by admitting department. Healthc Inform Res. 2014; 20(4):280–287.
Article12. Ahearn MD, Kerr SJ. General practitioners' perceptions of the pharmaceutical decision-support tools in their prescribing software. Med J Aust. 2003; 179(1):34–37.
Article13. Magnus D, Rodgers S, Avery AJ. GPs' views on computerized drug interaction alerts: questionnaire survey. J Clin Pharm Ther. 2002; 27(5):377–382.
Article14. Glassman PA, Simon B, Belperio P, Lanto A. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002; 40(12):1161–1171.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- National Rules for Drug–Drug Interactions: Are They Appropriate for Tertiary Hospitals?
- Drug Utilization Review (DUR) Policy of Government and Directivity
- Constructing a Real-Time Prescription Drug Monitoring System
- eEfforts to Improve Physicians Prescription in Developed Countries
- The Evaluation of Drug Utilization Review on Potentially Inappropriate Medications for Elderly Patients in a Tertiary Hospital