Obstet Gynecol Sci.
2016 Mar;59(2):123-129. 10.5468/ogs.2016.59.2.123.
Increased expression of nuclear factor kappa-B p65 subunit in adenomyosis
- Affiliations
-
- 1Department of Obstetrics and Gynecology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. kimsung@amc.seoul.kr
- 2Department of Obstetrics and Gynecology, Chungbuk National University College of Medicine, Cheongju, Korea.
- KMID: 2159007
- DOI: http://doi.org/10.5468/ogs.2016.59.2.123
Abstract
OBJECTIVE
Nuclear factor kappa-B (NF-κB) is a critical proinflammatory regulator that has been suggested to play a pivotal role in the pathogenesis and pathophysiology of endometriosis. In the present study, we aimed to evaluate whether the expression of NF-κB p65 subunit is increased in the eutopic endometrium and/or in the adenomyosis nodule of women with adenomyosis.
METHODS
Thirty-three women with histologically confirmed adenomyosis after laparoscopic or transabdominal hysterectomy were recruited. Women with carcinoma in situ of uterine cervix without evidence of adenomyosis or endometriosis (n=32) served as controls. Formalin-fixed, paraffin-embedded archival tissues were sectioned and immunostained utilizing a monoclonal anti-human NF-κB p65 subunit antibody, and the immunoreactivity of NF-κB p65 subunit was compared between women with and without adenomyosis.
RESULTS
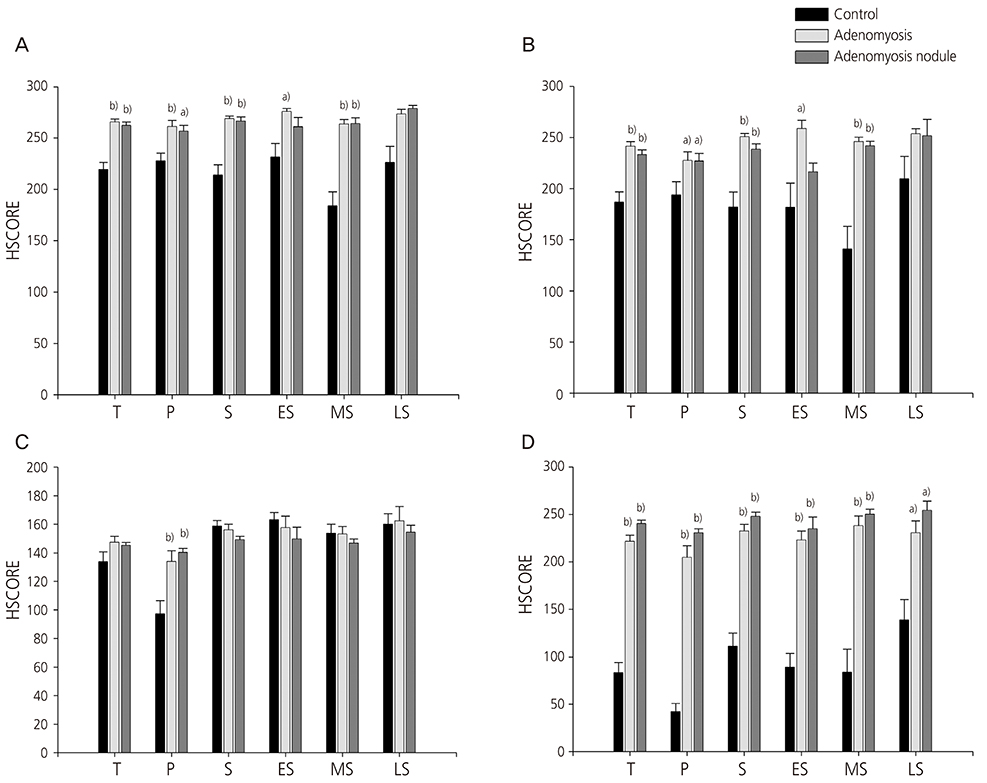
The immunoreactivities of both the nuclear and the cytoplasmic NF-κB p65 subunit were significantly increased in the stromal cells in the eutopic endometrium as well as in the adenomyosis nodule of women with adenomyosis compared with controls, respectively. The nuclear expression of NF-κB p65 subunit was significantly higher in the glandular cells in the eutopic endometrium as well as the adenomyosis nodule of women with adenomyosis compared with controls, respectively.
CONCLUSION
The expression of NF-κB p65 is increased in the eutopic endometrium and adenomyosis nodule of women with adenomyosis, which strongly suggest that NF-κB plays a critical role in the pathogenesis and/or pathophysiology of adenomyosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006; 20:511–521.2. Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006; 20:603–616.3. McElin TW, Bird CC. Adenomyosis of the uterus. Obstet Gynecol Annu. 1974; 3:425–441.4. Curtis KM, Hillis SD, Marchbanks PA, Peterson HB. Disruption of the endometrial-myometrial border during pregnancy as a risk factor for adenomyosis. Am J Obstet Gynecol. 2002; 187:543–544.5. Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod. 1997; 57:514–519.6. Ulukus M, Ulukus EC, Seval Y, Cinar O, Zheng W, Arici A. Expression of interleukin-8 receptors in patients with adenomyosis. Fertil Steril. 2006; 85:714–720.7. Wang F, Li H, Yang Z, Du X, Cui M, Wen Z. Expression of interleukin-10 in patients with adenomyosis. Fertil Steril. 2009; 91:1681–1685.8. Huang TS, Chen YJ, Chou TY, Chen CY, Li HY, Huang BS, et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med. 2014; 18:1358–1371.9. Streuli I, Dubuisson J, Santulli P, de Ziegler D, Batteux F, Chapron C. An update on the pharmacological management of adenomyosis. Expert Opin Pharmacother. 2014; 15:2347–2360.10. Ota H, Igarashi S, Hatazawa J, Tanaka T. Is adenomyosis an immune disease. Hum Reprod Update. 1998; 4:360–367.11. Zhou S, Yi T, Liu R, Bian C, Qi X, He X, et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteomics. 2012; 11:M112.12. Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012; 26:203–234.13. Gonzalez-Ramos R, Defrere S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012; 98:520–528.14. Kim SH, Ihm HJ, Oh YS, Chae HD, Kim CH, Kang BM. Increased nuclear expression of nuclear factor kappa-B p65 subunit in the eutopic endometrium and ovarian endometrioma of women with advanced stage endometriosis. Am J Reprod Immunol. 2013; 70:497–508.15. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975; 122:262–263.16. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012; 98:572–579.17. Vercellini P, Vigano P, Somigliana E, Daguati R, Abbiati A, Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006; 20:465–477.18. Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update. 2014; 20:386–402.19. Benagiano G, Brosens I. The endometrium in adenomyosis. Womens Health (Lond Engl). 2012; 8:301–312.20. Li B, Chen M, Liu X, Guo SW. Constitutive and tumor necrosis factor-α-induced activation of nuclear factor-κB in adenomyosis and its inhibition by andrographolide. Fertil Steril. 2013; 100:568–577.21. Kim SR, Kim SH, Lee HW, Chae HD, Kim CH, Kang BM. Increased expression of p21-activated kinase in adenomyosis. Fertil Steril. 2010; 94:1125–1128.22. Kim SH, Lee HW, Kim YH, Koo YH, Chae HD, Kim CH, et al. Down-regulation of p21-activated kinase 1 by progestin and its increased expression in the eutopic endometrium of women with endometriosis. Hum Reprod. 2009; 24:1133–1141.23. Yi KW, Kim SH, Ihm HJ, Oh YS, Chae HD, Kim CH, et al. Increased expression of p21-activated kinase 4 in adenomyosis and its regulation of matrix metalloproteinase-2 and -9 in endometrial cells. Fertil Steril. 2015; 103:1089–1097.e2.24. Nie J, Lu Y, Liu X, Guo SW. Immunoreactivity of progesterone receptor isoform B, nuclear factor kappaB, and IkappaBalpha in adenomyosis. Fertil Steril. 2009; 92:886–889.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Analysis of Nuclear Factor, p38, and Cyclin D1 Proteins in Premalignant Lesions and Carcinomas of the Colorectal Mucosa
- Subcellular localization of nuclear factor kappa B in term human fetal membranes and myometrium during labor
- Involvement of Nuclear Factor Kappa B in High-Fat Diet-Related Pancreatic Fibrosis in Rats
- Rectal cancer-derived exosomes activate the nuclear factor kappa B pathway and lung fibroblasts by delivering integrin beta-1
- Correlation between the Expression of Nuclear Factor-kappaB p65 Protein with the Expression of Nuclear Factor-kappaB p50 Protein and the Clinicopathologic Factors in Colorectal Cancer