Yonsei Med J.
2008 Oct;49(5):775-782. 10.3349/ymj.2008.49.5.775.
Docetaxel Chemotherapy of Korean Patients with Hormone-refractory Prostate Cancer: Comparative Analysis between 1st-line and 2nd-line Docetaxel
- Affiliations
-
- 1Urologic Oncology Clinic, National Cancer Center, Goyang, Korea. uroonco@ncc.re.kr
- KMID: 2158196
- DOI: http://doi.org/10.3349/ymj.2008.49.5.775
Abstract
- PURPOSE
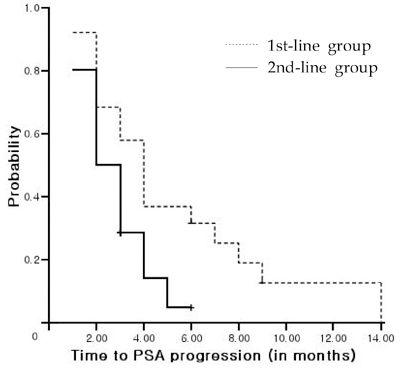
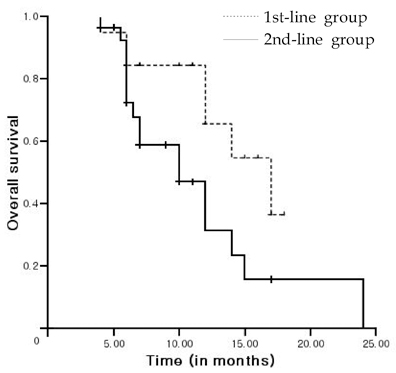
This study was undertaken to investigate the outcomes associated with docetaxel treatment of Korean patients with hormone-refractory prostate cancer (HRPC) and to compare its clinical efficacies in 1st and 2nd-line settings. PATIENTS AND METHODS: This study was retrospectively performed and included 47 patients with HRPC. The 1st-line group consisted of 19 patients who had not undergone prior chemotherapy, and the 2nd-line group consisted of 28 patients who underwent prior chemotherapy. All patients were treated with 75mg/m2 IV docetaxel every 3 weeks and 5mg of prednisone twice daily with a continuous androgen blockade. RESULTS: Of 47 study subjects, 14 patients (29.8%) had > or = 50% PSA decline from baseline. PSA response was more common in the 1st-line group, but this was not statistically different (42.1% vs. 21.4%, p = 0.114). After a median follow up of 11 months (range, 6-24 months), the 1st-line group showed a longer time to PSA progression (4 vs. 2 months, p = 0.015) and survival (17 vs. 10 months, p = 0.037) than the 2nd-line group. In terms of toxicities, no difference was apparent between the 2 groups. CONCLUSION: In a 1st-line setting, docetaxel is an effective and tolerable agent for Korean HRPC patients, and that its efficacy is limited, although 2nd-line docetaxel is tolerable.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Exponential Rise in Prostate-Specific Antigen (PSA) during Anti-Androgen Withdrawal Predicts PSA Flare after Docetaxel Chemotherapy in Patients with Castration-Resistant Prostate Cancer
Kyung Seok Han, Sung Joon Hong
Yonsei Med J. 2015;56(2):368-374. doi: 10.3349/ymj.2015.56.2.368.
Reference
-
1. Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001. 51:15–36.
Article2. Park SK, Sakoda LC, Kang D, Chokkalingam AP, Lee E, Shin HR, et al. Rising prostate cancer rates in South Korea. Prostate. 2006. 66:1285–1291.
Article3. McLeod DC. Hormonal therapy in the treatment of carcinoma of the prostate. Cancer. 1995. 75:Suppl 7. 1914–1919.
Article4. Huggins C, Hodges CV. Studies on prostatic cancer: I. the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972. 22:232–240.
Article5. Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989. 321:419–424.
Article6. Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998. 339:1036–1042.
Article7. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cacner. N Engl J Med. 2004. 351:1502–1512.
Article8. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004. 351:1513–1520.
Article9. Scher HI, Eisenberger M, D'Amico AV, Halabi S, Small EJ, Morris M, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004. 22:537–556.
Article10. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.11. Kreis W, Budman DR, Calabro A. Unique synergism or antagonism of combinations of chemotherapeutic and hormonal agents in human prostate cancer cell lines. Br J Urol. 1997. 79:196–202.
Article12. Díaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry. 1993. 32:2747–2755.
Article13. Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997. 57:229–233.14. Miyoshi Y, Uemura H, Nakamura M, Hasumi H, Sugiura S, Makiyama K, et al. Treatment of androgen-independent, hormone-refractory prostate cancer with docetaxel in Japanese patients. Int J Clin Oncol. 2005. 10:182–186.
Article15. Akaza H, Moore MA, Chang SJ, Cheng C, Choi HY, Esuvaranathan K, et al. The 5th conference on Asian Trends in prostate cancer hormone therapy. Asian Pac J Cancer Prev. 2007. 8:3–12.16. Joshua AM, Nordman I, Venkataswaran R, Clarke S, Stockler MR, Boyer MJ. Weekly docetaxel as second line treatment after mitozantrone for androgen-independent prostate cancer. Intern Med J. 2005. 35:468–472.
Article17. Michels J, Montermurro T, Murray N, Kollmannsberger C, Nguyen Chi K. First- and second line chemotherapy with docetaxel or mitoxantrone in patients with hormone-refractory prostate cancer: does sequence matter? Cancer. 2006. 106:1041–1046.18. Berthold DR, Sternberg CN, Tannock IF. Management of advanced prostate cancer after first-line chemotherapy. J Clin Oncol. 2005. 23:8247–8252.
Article19. Calabrò F, Sternberg CN. Current indications for chemotherapy in prostate cancer patients. Eur Urol. 2007. 51:17–26.
Article20. Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin. 2005. 55:300–318. quiz 323-5.
Article21. Mendiratta P, Armstrong AJ, George DJ. Current standard and investigational approaches to the management of hormone-refractory prostate cancer. Rev Urol. 2007. 9:Suppl 1. S9–S19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- What's New in Hormone-refractory Prostate Cancer Treatment
- Docetaxel rechallenge in metastatic castrationresistant prostate cancer: A retrospective, singlecenter study
- Comparison of Gefitinib versus Docetaxel in Patients with Pre-Treated Non-Small Cell Lung Cancer (NSCLC)
- Docetaxel as Second-line Monotherapy for Advanced Non-small Cell Lung Cancer