J Korean Med Sci.
2006 Aug;21(4):627-632. 10.3346/jkms.2006.21.4.627.
Granulocyte Antibodies in Korean Neonates with Neutropenia
- Affiliations
-
- 1Department of Laboratory Medicine, Inje University Sanggye Paik Hospital, Seoul, Korea. kscosby@sanggyepaik.ac.kr
- 2Department of Pediactrics, Inje University Sanggye Paik Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, College of Medicine, Seoul National University, Seoul, Korea.
- KMID: 2157812
- DOI: http://doi.org/10.3346/jkms.2006.21.4.627
Abstract
- Neonatal alloimmune neutropenia (NAN) is a disease that can cause severe and prolonged neutropenia in neonates. However, no report is available on the incidence of granulocyte antibody in neonates, the target antigen of this antibody, and the estimated incidence of NAN in Korea. Among a total of 856 neonates admitted to a neonatal intensive care unit (NICU) over a five year period, a total of 105 neonates with neutropenia were enrolled in this study. Positive reactions were observed in the sera of six neonates (5.7%, 6/105) by mixed passive hemagglutination assay (MPHA). To confirm the presence of NAN, MPHA and granulocyte antigen typing (HNA-1a, -1b, -2a, -4a, and -5a) were performed on neonatal and maternal blood. To differentiate granulocyte antibody and HLA antibody, MPHA was also performed using HLA antibody adsorbed serum. We confirmed three cases (2.9%, 3/105) of NAN among neonates with neutropenia in which granulocyte antibody specificities (two anti-HNA-1b and one anti-HNA-1a) and fetomaternal granulocyte antigen mismatches were identified. In this study, the estimated incidence of NAN was 0.35% (3/856) among neonates admitted to NICUs in Korea.
MeSH Terms
-
Polymerase Chain Reaction/methods
Neutropenia/blood/diagnosis/*immunology
Korea
Isoantigens/genetics/immunology
Isoantibodies/*immunology
Intensive Care Units, Neonatal/statistics & numerical data
Infant, Newborn
Humans
Hemagglutination Tests
HLA Antigens/immunology
Granulocytes/*immunology
Genotype
Antibody Specificity/immunology
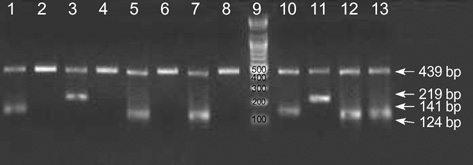
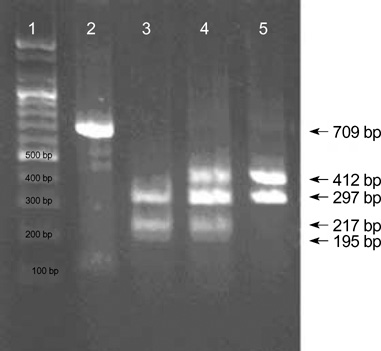
Figure
Cited by 1 articles
-
Antibiotic-induced Severe Neutropenia with Multidrug-Dependent Antineutrophil Antibodies Developed in A Child with Streptococcus pneumoniae Infection
Young-Ho Lee, Ha-Baik Lee, Jung-Yun Kim, Yeon-Jung Lim, Su-A Shin, Tae-Hee Han
J Korean Med Sci. 2009;24(5):975-978. doi: 10.3346/jkms.2009.24.5.975.
Reference
-
1. Maheshwari A, Christensen RD, Calhoun DA. Immune-mediated neutropenia in the neonate. Acta Paediatr Suppl. 2002. 91:98–103.
Article2. Christensen RD, Calhoun DA, Rimsza LM. A practical approach evaluating and treating neutropenia in the neonatal intensive care unit. Clin Perinatol. 2000. 27:577–601.3. Maheshwari A, Christensen RD, Calhoun DA. Resistance to recombinant human granulocyte colony-stimulating factor in neonatal alloimmune neutropenia associated with anti-human neutrophil antigen-2a (NB1) antibodies. Pediatrics. 2002. 109:E64.
Article4. Gilmore MM, Stroncek DF, Korones DN. Treatment of alloimmune neonatal neutropenia with granulocyte colony-stimulating factor. J Pediatr. 1994. 125:948–951.
Article5. Zupanska B, Uhrynowska M, Guz K, Maslanka K, Brojer E, Czestynska M, Radomska I. The risk of antibody formation against HNA1a and HNA1b granulocyte antigens during pregnancy and its relation to neonatal neutropenia. Transfus Med. 2001. 11:377–382.
Article6. Levine DH, Madyastha PR. Isoimmune neonatal neutropenia. Am Perinatol. 1986. 3:231–233.
Article7. Skacel PO, Stacey TE, Tidmarsh CE, Contreras M. Maternal alloimmunization to HLA, platelet and granulocyte-specific antigens during pregnancy: its influence on cord blood granulocyte and platelet counts. Br J Haematol. 1989. 71:119–123.
Article8. Bux J, Jung KD, Kauth T, Mueller-Eckhardt C. Serological and clinical aspects of granulocyte antibodies leading to alloimmune neonatal neutropenia. Transfus Med. 1992. 2:143–149.
Article9. Bux J, Chapman J. Report on the second international granulocyte serology workshop. Transfusion. 1997. 37:977–983.
Article10. Hagimoto R, Koike K, Sakashita K, Ishida T, Nakazawa Y, Kurokawa Y, Kamijo T, Saito S, Hiraoka A, Kobayashi M, Komiyama A. A possible role for maternal HLA antibody in a case of alloimmune neonatal neutropenia. Transfusion. 2001. 41:615–620.11. Stroncek D. Granulocyte antigens and antibody detection. Vox Sang. 2004. 87:91–94.12. Araki N, Nose Y, Kohsaki M, Mito H, Ito K. Anti-granulocyte antibody screening with extracted granulocyte antigens by a micro-mixed passive hemagglutination method. Vox Sang. 1999. 77:44–51.
Article13. Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979. 95:89–98.14. Mouzinho A, Rosenfeld CR, Sanchez PJ, Risser R. Revised reference ranges for circulating neutrophils in very-low-birth-weight neonates. Pediatrics. 1994. 94:76–82.15. Helmerhorst FM, van Oss CJ, Bruynes EC, Engelfriet CP, von dem Borne AE. Elution of granulocyte and platelet antibodies. Vox Sang. 1982. 43:196–204.
Article16. Bux J, Stein EL, Santoso S, Mueller-Eckhardt C. NA gene frequencies in the German population, determined by polymerase chain reaction with sequence-specific primers. Transfusion. 1995. 35:54–57.
Article17. Clague HD, Fung YL, Minchinton RM. Human neutrophil antigen-4a gene frequencies in an Australian population, determined by new polymerase chain reaction method using sequence-specific primers. Transfus Med. 2003. 13:149–152.18. Simsek S, van der Schoot CE, Daams M, Huiskes E, Clay M, McCullough J, van Dalen C, Stroncek D, von dem Borne AE. Molecular characterization of antigenic polymorphisms (Onds(a) and Mart(a)) of the beta 2 family recognized by human leukocyte alloantisera. Blood. 1996. 88:1350–1358.19. Lucas GF, Metcalfe P. Platelet and granulocyte glycoprotein polymorphisms. Transfus Med. 2000. 10:157–174.
Article20. Han KS, Um TH. Frequency of neutrophil-specific antigens among Koreans using the granulocyte indirect immunofluorescence test (GIFT). Immunohematology. 1997. 13:15–16.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alloimmunization to Granulocyte Antigens among Korean Pregnant Women
- Frequency of Granulocyte-Spedfic Anhgens among Koreans
- Therapeutic Effect of Different Doses of Recombinant Human Granulocyte Colony-Stimulating Factor(rhG-CSF) on Neonatal Sepsis Complicated by Neutropenia
- Kostmann's Disease: A Case Report
- Two Cases of Autoimmune Neutropenia of Infancy