Ann Dermatol.
2009 Nov;21(4):382-388. 10.5021/ad.2009.21.4.382.
The Expression of NMDA Receptor 1 Correlates with Clinicopathological Parameters in Cutaneous Squamous Cell Carcinoma
- Affiliations
-
- 1Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea. yymmpark@hotmail.com
- KMID: 2156501
- DOI: http://doi.org/10.5021/ad.2009.21.4.382
Abstract
- BACKGROUND
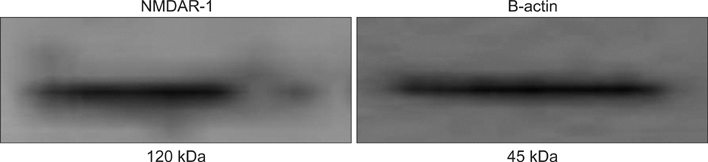
Ionotropic glutamate receptors of the N-methyl-D-aspartate receptor (NMDAR) type are expressed on keratinocytes and play a role in the proliferation, differentiation, and cornification of keratinocytes. However, the expression profile of NMDAR and its role in cutaneous malignancy is unclear.
OBJECTIVE
We analyzed the expression of NMDAR-1 in cutaneous squamous cell carcinoma (SCC) and investigated the relationship between NMDAR-1 expression and clinicopathological parameters.
METHODS
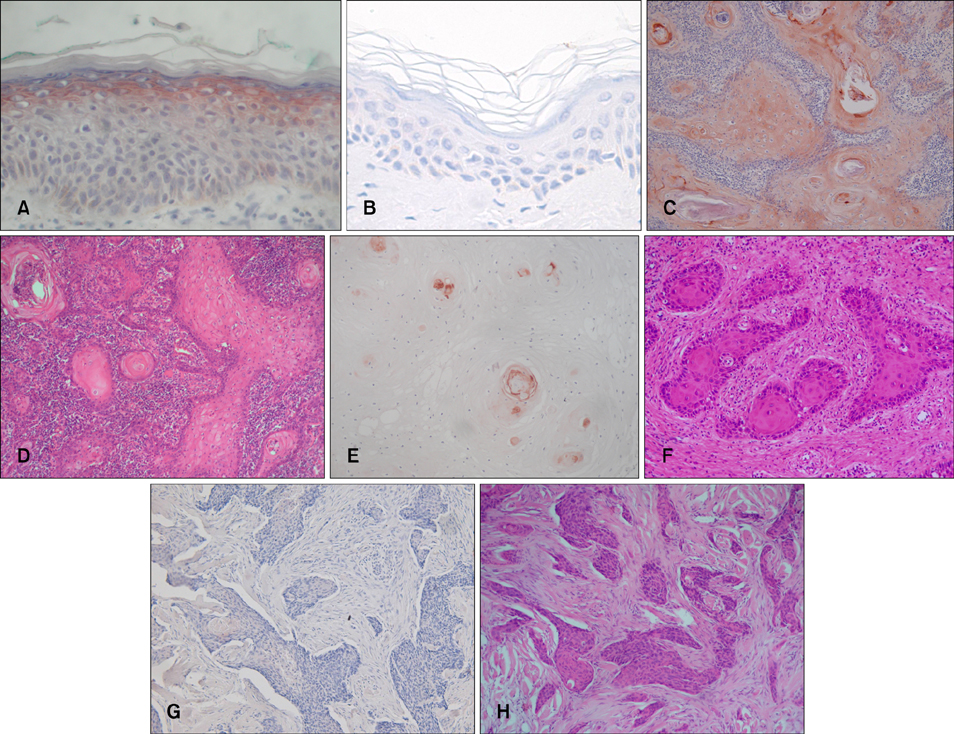
Thirty-two patients with biopsy-proven cutaneous SCC were enrolled in this study. Each patient was analyzed for tumor diameter, location, local recurrence, and metastasis by conducting a chart review. The SCC specimens were histologically divided into differentiated and undifferentiated groups based on Broders' system. NMDAR-1 expression was examined by performing immunohistochemistry, and the relative staining intensity in the SCCs was graded into 5 levels. According to the staining intensity of NMDAR-1, the specimens were categorized into two groups: the higher group and the lower group.
RESULTS
Fifteen (88%) of 17 tumors in the higher group were differentiated SCC, whereas 14 (93%) of 15 tumors in the lower group were undifferentiated SCC. In addition, NMDAR-1 expression was inversely correlated with metastasis (p=0.049). Local recurrence was associated with a lower staining intensity, but the results were not statistically significant.
CONCLUSION
Our results demonstrate that NMDAR-1 expression in cutaneous SCC is significantly correlated with its differentiation and metastasis. Therefore, it may be a prognostic indicator for cutaneous SCC.
Keyword
MeSH Terms
Figure
Reference
-
1. Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci U S A. 2001. 98:6372–6377.
Article2. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000. 130:1007S–1015S.
Article3. Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981. 21:165–204.
Article4. Foldes RL, Rampersad V, Kamboj RK. Cloning and sequence analysis of cDNAs encoding human hippocampus N-methyl-D-aspartate receptor subunits: evidence for alternative RNA splicing. Gene. 1993. 131:293–298.
Article5. Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001. 22:174–181.
Article6. Nahm WK, Philpot BD, Adams MM, Badiavas EV, Zhou LH, Butmarc J, et al. Significance of N-methyl-D-aspartate (NMDA) receptor-mediated signaling in human keratinocytes. J Cell Physiol. 2004. 200:309–317.
Article7. Fischer M, Glanz D, William T, Klapperstuck T, Wohlrab J, Marsch W. N-methyl-D-aspartate receptors influence the intracellular calcium concentration of keratinocytes. Exp Dermatol. 2004. 13:512–519.
Article8. Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol. 1991. 127:57–63.
Article9. Fischer M, William T, Helmbold P, Wohlrab J, Marsch W. Expression of epidermal N-methyl-D-aspartate receptors (NMDAR1) depends on formation of the granular layer--analysis in diseases with parakeratotic cornification. Arch Dermatol Res. 2004. 296:157–162.
Article10. North WG, Fay MJ, Du J, Cleary M, Gallagher JD, McCann FV. Presence of functional NMDA receptors in a human neuroblastoma cell line. Mol Chem Neuropathol. 1997. 30:77–94.
Article11. Yoshioka A, Ikegaki N, Williams M, Pleasure D. Expression of N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor genes in neuroblastoma, medulloblastoma, and other cells lines. J Neurosci Res. 1996. 46:164–178.12. Broders AC. Squamous-cell epithelioma of the skin: a study of 256 cases. Ann Surg. 1921. 73:141–160.
Article13. Maatkamp A, Vlug A, Haasdijk E, Troost D, French PJ, Jaarsma D. Decrease of Hsp25 protein expression precedes degeneration of motoneurons in ALS-SOD1 mice. Eur J Neurosci. 2004. 20:14–28.
Article14. Choi SW, Park SY, Hong SP, Pai H, Choi JY, Kim SG. The expression of NMDA receptor 1 is associated with clinicopathological parameters and prognosis in the oral squamous cell carcinoma. J Oral Pathol Med. 2004. 33:533–537.
Article15. Barksdale SK, O'Connor N, Barnhill R. Prognostic factors for cutaneous squamous cell and basal cell carcinoma. Determinants of risk of recurrence, metastasis, and development of subsequent skin cancers. Surg Oncol Clin N Am. 1997. 6:625–638.
Article16. Johnson TM, Rowe DE, Nelson BR, Swanson NA. Squamous cell carcinoma of the skin (excluding lip and oral mucosa). J Am Acad Dermatol. 1992. 26:467–484.
Article17. Kwa RE, Campana K, Moy RL. Biology of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992. 26:1–26.
Article18. Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992. 26:976–990.
Article19. Bernstein SC, Lim KK, Brodland DG, Heidelberg KA. The many faces of squamous cell carcinoma. Dermatol Surg. 1996. 22:243–254.
Article20. Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001. 344:975–983.
Article21. Eriksen JG, Steiniche T, Askaa J, Alsner J, Overgaard J. The prognostic value of epidermal growth factor receptor is related to tumor differentiation and the overall treatment time of radiotherapy in squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2004. 58:561–566.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Expression of PD-L1 in Cutaneous Squamous Cell Carcinoma and Its Association with Risk of Metastasis
- Cutaneous Metastasis of Esophageal Squamous Cell Carcinoma Mimicking Benign Soft Tissue Tumor
- Expression of Minichromosome Maintenance Protein 7 and Smad 4 in Squamous Cell Carcinoma of the Esophagus
- A Case of Metastatic Cutaneous Squamous Cell Carcinoma Arising in Chronic Osteomyelitic Focus
- Pseudoangiosarcomatous Squamous Cell Carcinoma of the Face