J Vet Sci.
2014 Sep;15(3):433-437. 10.4142/jvs.2014.15.3.433.
Effects of progestagen exposure duration on estrus synchronization and conception rates of crossbreed ewes undergoing fixed time artificial insemination
- Affiliations
-
- 1Laboratory of Animal Reproduction, Londrina State University, Londrina PR 86051-990, Brazil. mseneda@uel.br
- 2Department of Animal Science, McGill University, Quebec H9X 3V9, Canada.
- KMID: 2155628
- DOI: http://doi.org/10.4142/jvs.2014.15.3.433
Abstract
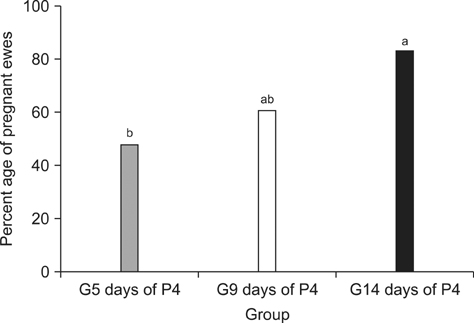
- Synchronization of estrus and ovulation are of paramount importance in modern livestock improvement programs. These methods are critical for assisted reproduction technologies, including artificial insemination and embryo transfer, that can increase productivity. In the current study, subcutaneous implants containing norgestomet were placed for long (14 days), medium (9 days), and short (5 days) periods of time in 70 crossbred ewes undergoing fixed-time artificial insemination. The resulting effects on estrus synchronization and conception rates were subsequently evaluated. Among the synchronized ewes, 85.7% (60/70) underwent estrus over a period of 72 h after progestagen treatment ceased. The shortest mean interval between withdrawal of the device and onset of estrus (34.2 +/- 8.9 h) was observed in the G14 days of P4 group (p < 0.05). The conception rate of the G14 days of P4 group was statistically higher than that of the other groups (83.3% vs. 60.9% vs. 47.8%; p < 0.05). In conclusion, 14 days of norgestomet treatment produced higher conception rates and a greater number of pregnancies at the beginning of the breeding season.
Keyword
MeSH Terms
Figure
Reference
-
1. Abecia JA, Forcada F, González-Bulnes A. Hormonal control of reproduction in small ruminants. Anim Reprod Sci. 2012; 130:173–179.
Article2. Abecia JA, Forcada F, González-Bulnes A. Pharmaceutical control of reproduction in sheep and goats. Vet Clin North Am Food Anim Pract. 2011; 27:67–79.
Article3. Aboul-Naga AM, Aboul-Ela MB. Oestrous activity of Egyptian fat-tailed Ossimi ewes raised at different locations. J Agric Sci. 1984; 103:481–486.
Article4. Awel H, Eshetu L, Tadesse G, Birhanu A, Khar SK. Estrus synchronization in sheep with synthetic progestagens. Trop Anim Health Prod. 2009; 41:1521–1524.
Article5. Baldassarre H, Karatzas CN. Advanced assisted reproduction technologies (ATR) in goats. Anim Reprod Sci. 2004; 82-83:255–266.6. Binelli M, Thatcher WW, Mattos R, Baruselli PS. Antiluteolytic strategies to improve fertility in cattle. Theriogenology. 2001; 56:1451–1463.
Article7. Cox JF, Allende R, Lara E, Leiva A, Díaz T, Dorado J, Saravia F. Follicular dynamics, interval to ovulation and fertility after AI in short-term progesterone and PGF2α oestrous synchronization protocol in sheep. Reprod Domest Anim. 2012; 47:946–951.
Article8. Fox J, Weisberg S. An R Companion to Applied Regression. 2nd ed. Thousand Oaks: SAGE Publications;2011.9. King ME, McKelvey WAC, Dingwall WS, Matthews KP, Gebbie FE, Mylne MJA, Stewart E, Robinson JJ. Lambing rates and litter sizes following intrauterine or cervical insemination of frozen/thawed semen with or without oxytocin administration. Theriogenology. 2004; 62:1236–1244.
Article10. Knights M, Ramgattie R, Siew N, Singh-Knights D, Bourne G. Effectiveness of a short-term treatment with progesterone injections on synchrony of lambing and fertility in tropical hair sheep. Anim Reprod Sci. 2011; 126:70–75.
Article11. Koyuncu M, Alticekic SO. Effects of progestagen and Pmsg on estrous synchronization and fertility in Kivircik ewes during natural breeding season. Asian-Australas J Anim Sci. 2010; 23:308–311.
Article12. Martemucci G, D'Alessandro AG. Estrous and fertility responses of dairy ewes synchronized with combined short term GnRH, PGF2α and estradiol benzoate treatments. Small Rumin Res. 2010; 93:41–47.
Article13. Martins LHS, da Silva Dranca G, de Medeiros Bastos G, Prado OR, Saab BB. Ovine estrus synchronization with short or long-term medroxiprogesterone acetate exposure to laparoscopic insemination with frozen-thawed semen. Synergismus scyentifica UTFPR. 2012; 7(1):14. Maxwell WMC, Hewitt LJ. A comparision of vaginal, cervical and intrauterine insemination of sheep. J Agric Sci. 1986; 106:191–193.
Article15. Paulenz H, Söderquist L, Adnøy T, Nordstoga AB, Andersen Berg K. Effect of vaginal and cervical deposition of semen on the fertility of sheep inseminated with frozen-thawed semen. Vet Rec. 2005; 156:372–375.
Article16. Pope WF, Cárdenas H. Sensitivity of sheep to exogenous prostaglandin F2α early in the estrous cycle. Small Rumin Res. 2004; 55:245–248.
Article17. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing;2013.18. Russel AJF, Doney JM, Gunn RG. Subjective assessment of body fat in live sheep. J Agric Sci. 1969; 72:451–454.
Article19. Salehi R, Kohram H, Towhidi A, Kermani Moakha H, Honarvar M. Follicular development and ovulation rate following different superovulatory treatments in Chall ewes. Small Rumin Res. 2010; 93:213–217.
Article20. Talebkhan Garoussi M, Farzaneh N, Gallehdar E, Mohri M. Reproductive performance in out-of-breeding season of fatty ewes using implant norgestomet with or without PMSG. Trop Anim Health Prod. 2012; 44:965–968.
Article21. Vilariño M, Rubianes E, Menchaca A. Ovarian responses and pregnancy rate with previously used intravaginal progesterone releasing devices for fixed-time artificial insemination in sheep. Theriogenology. 2013; 79:206–210.
Article22. Viñoles C, Forsberg M, Banchero G, Rubianes E. Effect of long-term and short-term progestagen treatment on follicular development and pregnancy rate in cyclic ewes. Theriogenology. 2001; 55:993–1004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of category-heifers, primiparous and multiparous lactating cows-in a large-scale resynchronization fixed-time artificial insemination program
- Use of a domestic Korean black goat (Capra hircus coreanae) with its chest crayon-harnessed in detecting estrus of Himalayan tahrs (Hemitragus jemlahicus)
- Factors affecting the success of resynchronization protocols with or without progesterone supplementation in dairy cows
- Alginate encapsulation preserves the quality and fertilizing ability of Mediterranean Italian water buffalo (Bubalus bubalis) and Holstein Friesian (Bos taurus) spermatozoa after cryopreservation
- Birth of puppies after intrauterine and intratubal insemination with frozen-thawed canine semen