Korean J Radiol.
2015 Jun;16(3):593-603. 10.3348/kjr.2015.16.3.593.
Interventional Radiological Treatment of Renal Transplant Complications: A Pictorial Review
- Affiliations
-
- 1Department of Radiological Sciences, Institute of Radiology, "A. Gemelli" Hospital - Catholic University, Rome 00168, Italy. roberto.iezzi.md@gmail.com
- 2Department of Surgical Science, Renal Transplantation Unit, "A. Gemelli" Hospital - Catholic University, Rome 00168, Italy.
- KMID: 2155529
- DOI: http://doi.org/10.3348/kjr.2015.16.3.593
Abstract
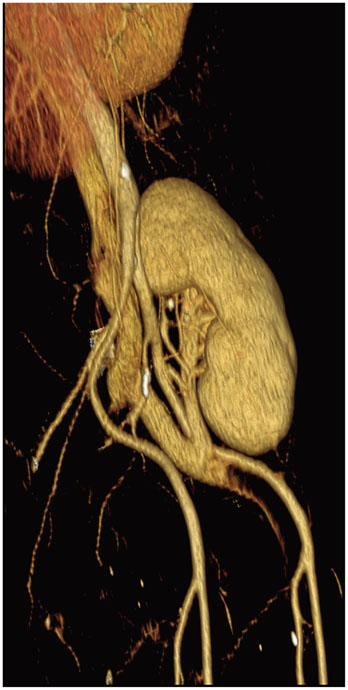
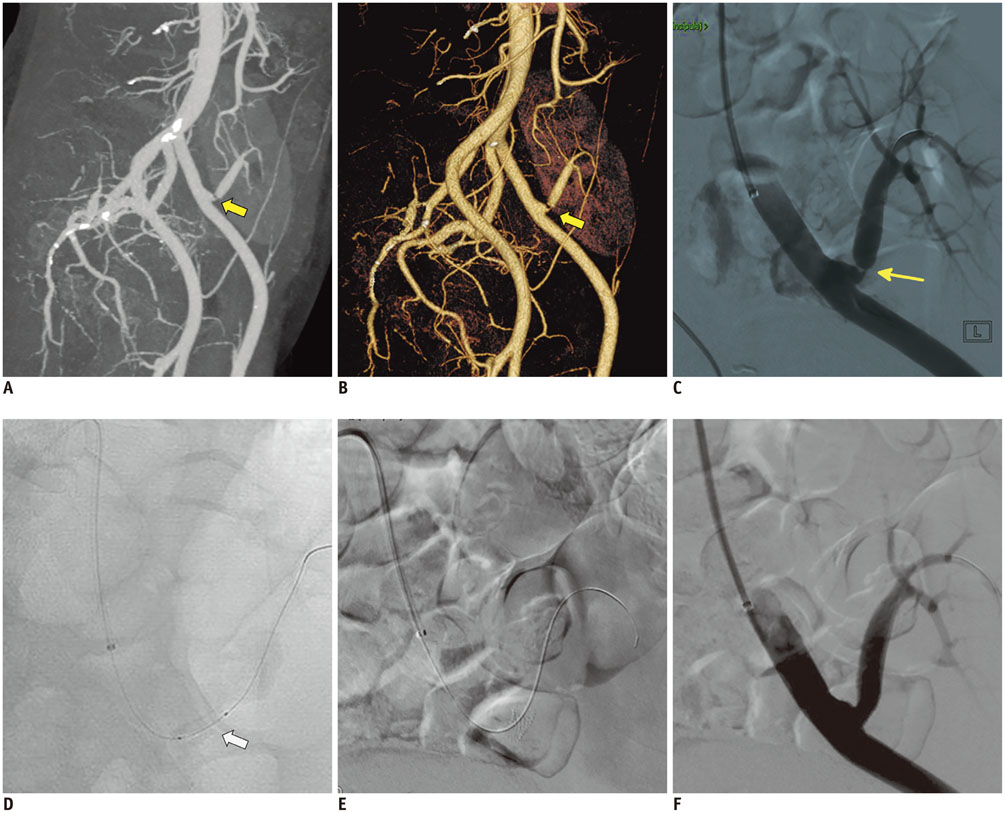
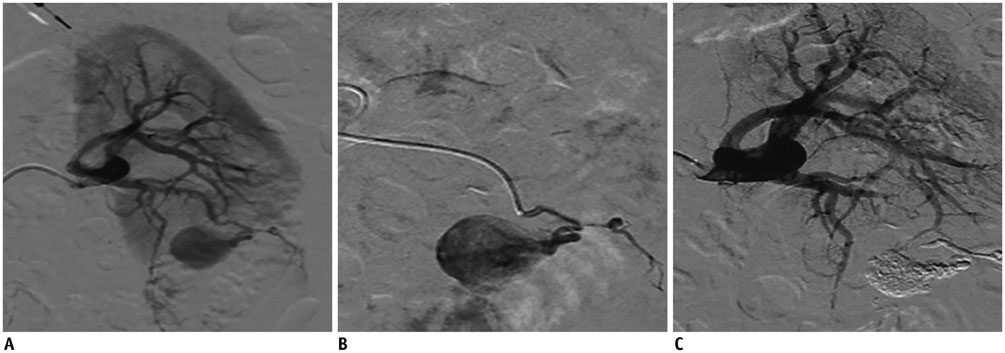
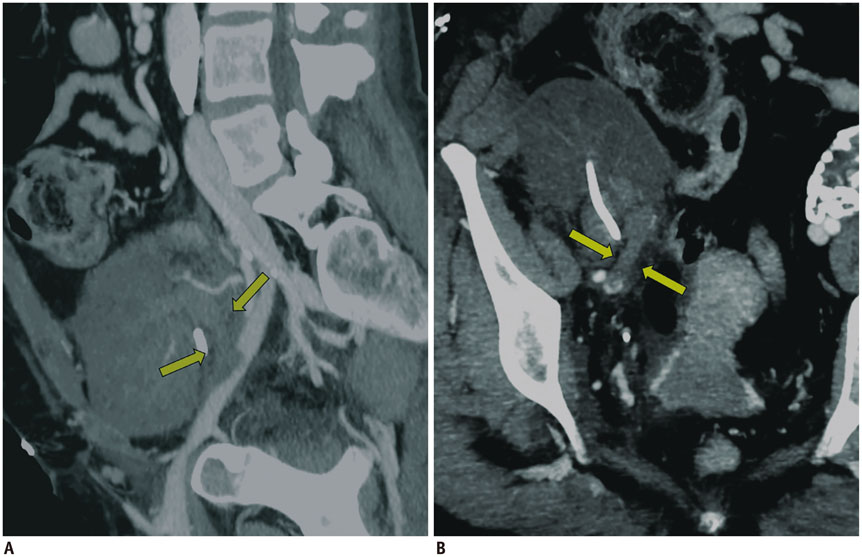
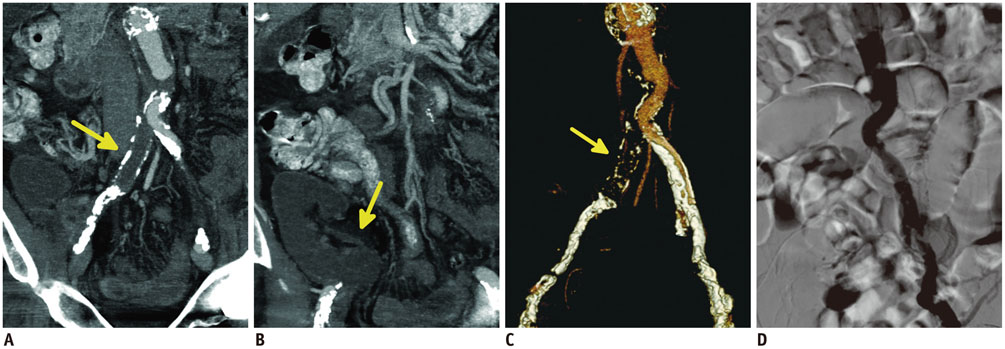
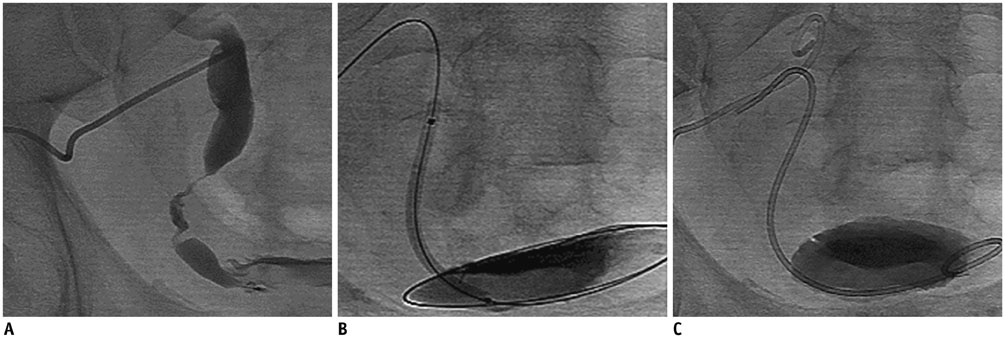
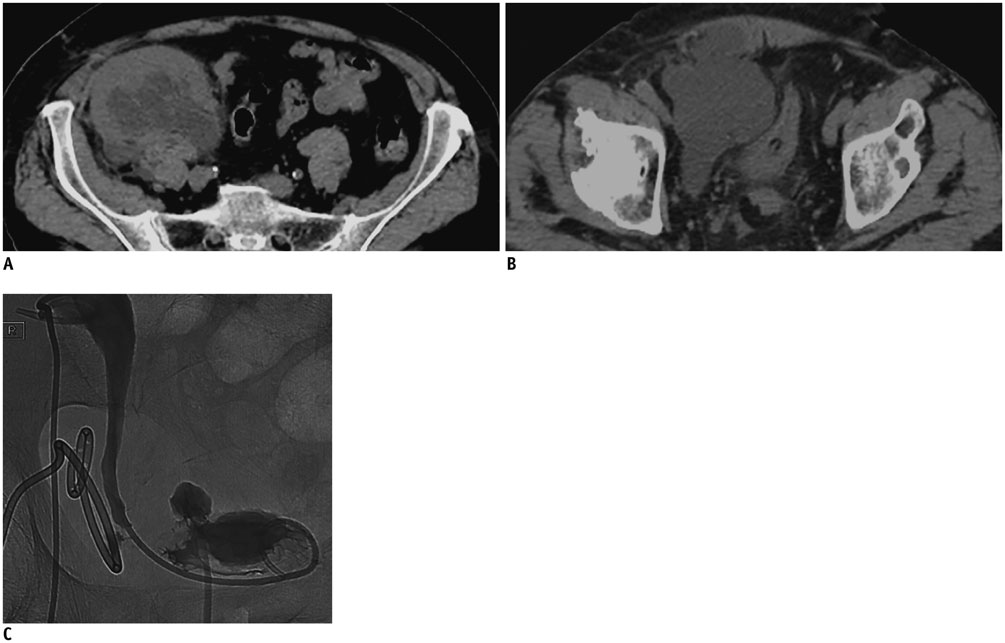
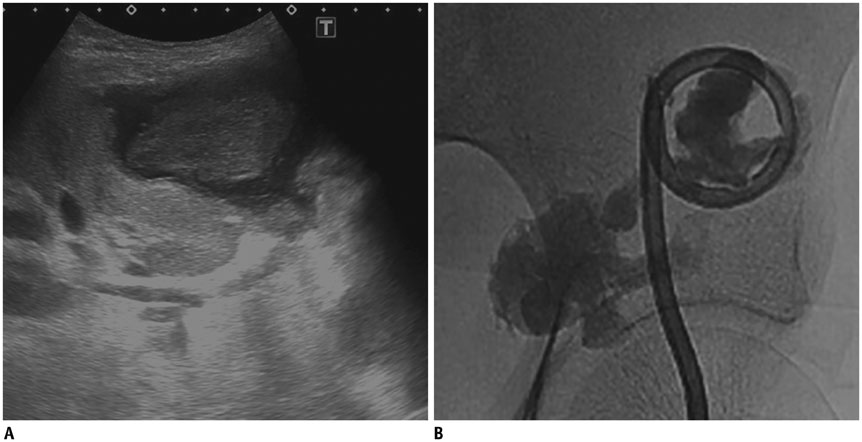
- Renal transplantation is the treatment of choice for patients with chronic renal failure, which produces a dramatic improvement in the quality of life and survival rates, in comparison to long-term dialysis. Nowadays, new imaging modalities allow early diagnosis of complications, and thanks to the recent developments of interventional techniques, surgery may be avoided in most cases. Knowledge in the types of renal transplant complications is fundamental for a correct pre-operative planning. In this article, we described the most common or clinically relevant renal transplant complications and explained their interventional management.
MeSH Terms
Figure
Reference
-
1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999; 341:1725–1730.2. Patel NH, Jindal RM, Wilkin T, Rose S, Johnson MS, Shah H, et al. Renal arterial stenosis in renal allografts: retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology. 2001; 219:663–667.3. Matheus WE, Reis LO, Ferreira U, Mazzali M, Denardi F, Leitao VA, et al. Kidney transplant anastomosis: internal or external iliac artery? Urol J. 2009; 6:260–266.4. Rundback JH, Rizvi A, Tomasula J. Percutaneous treatment of transplant renal artery stenosis: techniques and results. Tech Vasc Interv Radiol. 1999; 2:91–97.5. Salvadori M, Di Maria L, Rosati A, Larti A, Piperno R, Becherelli P, et al. Efficacy and safety of Palmaz stent implantation in the treatment of renal artery stenosis in renal transplantation. Transplant Proc. 2005; 37:1047–1048.6. Peregrin JH, Bürgelová M. Restoration of failed renal graft function after successful angioplasty of pressure-resistant renal artery stenosis using a cutting balloon: a case report. Cardiovasc Intervent Radiol. 2009; 32:548–553.7. Hedegard W, Saad WE, Davies MG. Management of vascular and nonvascular complications after renal transplantation. Tech Vasc Interv Radiol. 2009; 12:240–262.8. Dimitroulis D, Bokos J, Zavos G, Nikiteas N, Karidis NP, Katsaronis P, et al. Vascular complications in renal transplantation: a single-center experience in 1367 renal transplantations and review of the literature. Transplant Proc. 2009; 41:1609–1614.9. Flechner SM, Novick AC. Renal transplantation. In : Gillenwater JY, Grayhack JT, Howards SS, Mitchell ME, editors. Adult and Pediatric Urology. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins;2002. p. 941–954.10. Chen CH, Chen CH, Hsieh SR, Shu KH, Ho HC. Salvage of external iliac artery dissection immediately after renal transplant. Exp Clin Transplant. 2013; 11:274–277.11. Grenier N, Claudon M, Trillaud H, Douws C, Levantal O. Noninvasive radiology of vascular complications in renal transplantation. Eur Radiol. 1997; 7:385–391.12. Maleux G, Messiaen T, Stockx L, Vanrenterghem Y, Wilms G. Transcatheter embolization of biopsy-related vascular injuries in renal allografts. Long-term technical, clinical and biochemical results. Acta Radiol. 2003; 44:13–17.13. Ojo AO, Hanson JA, Wolfe RA, Agodoa LY, Leavey SF, Leichtman A, et al. Dialysis modality and the risk of allograft thrombosis in adult renal transplant recipients. Kidney Int. 1999; 55:1952–1960.14. Basić D, Hadzi-Djokić J, Milutinović D, Djokić M. [Vascular complications after kidney transplantation]. Srp Arh Celok Lek. 2003; 131:215–220.15. Rouvière O, Berger P, Béziat C, Garnier JL, Lefrançois N, Martin X, et al. Acute thrombosis of renal transplant artery: graft salvage by means of intra-arterial fibrinolysis. Transplantation. 2002; 73:403–409.16. Kaskarelis I, Koukoulaki M, Georgantas T, Bairamidis E, Kokkinos C, Ieronymou M, et al. Ureteral complications in renal transplant recipients successfully treated with interventional radiology. Transplant Proc. 2008; 40:3170–3172.17. Bhagat VJ, Gordon RL, Osorio RW, LaBerge JM, Kerlan RK Jr, Melzer JS, et al. Ureteral obstructions and leaks after renal transplantation: outcome of percutaneous antegrade ureteral stent placement in 44 patients. Radiology. 1998; 209:159–167.18. Yap R, Madrazo B, Oh HK, Dienst SG. Perirenal fluid collection after renal transplant. Am Surg. 1981; 47:287–290.19. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000; 342:605–612.20. Schwerk WB, Dürr HK. Ultrasound gray-scale pattern and guided aspiration puncture of abdominal abscesses. J Clin Ultrasound. 1981; 9:389–396.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neurological Complications Following Liver Transplant: A Pictorial Review of Radiological and Clinical Findings
- The Utility of 64 Channel Multidetector CT Angiography for Evaluating the Renal Vascular Anatomy and Possible Variations: a Pictorial Essay
- Renal embolization for trauma: a narrative review
- The Role of Interventional Radiology in Treatment of Patients with Acute Trauma: A Pictorial Essay
- Clinical Findings and Interventional Treatment of Gastrointestinal Fistulae: Pictorial Essay