J Korean Med Sci.
2015 Jan;30(1):16-23. 10.3346/jkms.2015.30.1.16.
Clinicopathologic Impacts of Poorly Differentiated Cluster-Based Grading System in Colorectal Carcinoma
- Affiliations
-
- 1Department of Pathology, Hallym University College of Medicine, Kangnam Sacred Heart Hospital, Seoul, Korea. jwkim@hallym.or.kr
- 2Department of Surgery, Hallym University College of Medicine, Kangnam Sacred Heart Hospital, Seoul, Korea.
- KMID: 2155443
- DOI: http://doi.org/10.3346/jkms.2015.30.1.16
Abstract
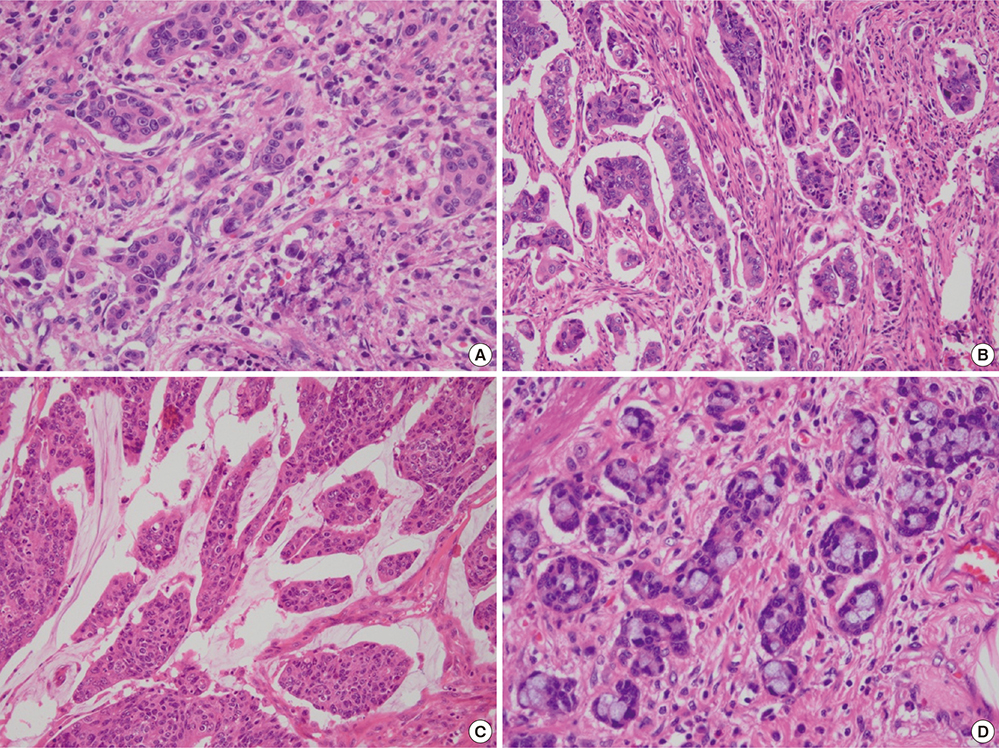
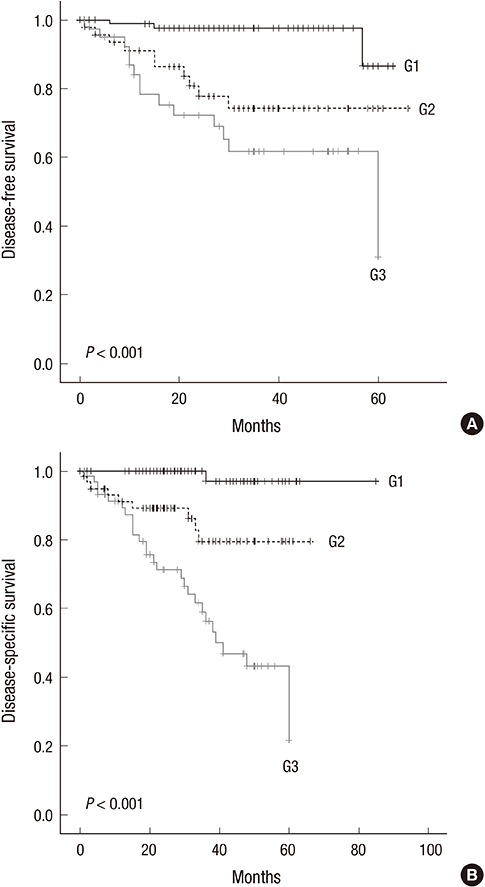
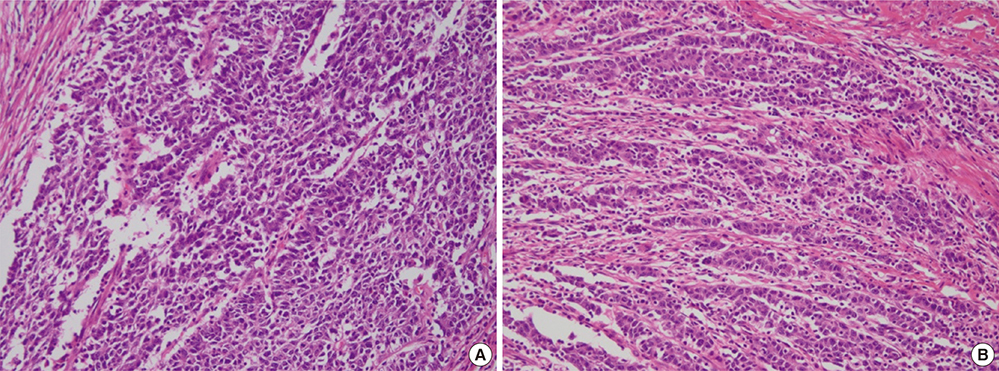
- Differentiation-based histologic grading of colorectal carcinoma (CRC) is widely used, but its clinical impact is limited by insufficient prognostic value, interobserver disagreement, and the difficulty of its application to CRC with specific histologic types such as mucinous and medullary carcinoma. A recently proposed novel grading system based on quantifying poorly differentiated clusters (PDCs) claims to have the advantages of reproducibility and improved prognostic value, and might apply to heterogeneous CRC. We aimed to validate the clinicopathologic significance of the PDCs-based grading system and to determine the relationship between this grading system and microsatellite instability (MSI). Two hundred and one patients who had undergone radical surgery were reviewed. Based on the number of PDCs, 85, 58, and 58 tumors were classified as grade (G) 1 (42.3%), G2 (28.9%), and G3 (28.9%), respectively. PDCs-based grade was significantly associated with T, N, and M stages; lymphovascular invasion; conventional histologic grade; and frequent tumor budding (all P <0.001). In multivariate analysis, PDCs-based grade was found to be an independent prognostic factor for disease-free survival (P = 0.022; hazard ratio, 3.709 [G2], 7.461 [G3]). G3 CRC significantly correlated with high MSI (MSI-H) compared to G1 and G2 (P = 0.002; odds ratio, 5.750). In conclusion, this novel grading would provide valuable prognostic information to a greater number of patients and would require continued verification. PDCs-based grading is feasible for CRCs with heterogeneous morphology, and we propose that the association between G3 and MSI-H be further evaluated in different histological subtypes of CRC.
MeSH Terms
Figure
Reference
-
1. Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura S-I, Quirke P, Riboli E, Sobin LH. Carcinoma of the colon and rectum. In : Bosman FT, editor. World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer;2010. p. 134–146.2. Thomas GD, Dixon MF, Smeeton NC, Williams NS. Observer variation in the histological grading of rectal carcinoma. J Clin Pathol. 1983; 36:385–391.3. Chandler I, Houlston RS. Interobserver agreement in grading of colorectal cancers-findings from a nationwide web-based survey of histopathologists. Histopathology. 2008; 52:494–499.4. Redston M. Epithelial neoplasms of the large intestine. In : Odze RD, Goldblum JR, editors. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. 2nd ed. Philadelphia, PA: Saunders Elsevier;2009. p. 621.5. Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012; 23:2479–2516.6. Prall F, Nizze H, Barten M. Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology. 2005; 47:17–24.7. Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, Katsurada Y, Nakamura T, Mochizuki H, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012; 36:193–201.8. Barresi V, Reggiani Bonetti L, Branca G, Di Gregorio C, Ponz de Leon M, Tuccari G. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch. 2012; 461:621–628.9. Ueno H, Hase K, Hashiguchi Y, Shimazaki H, Tanaka M, Miyake O, Masaki T, Shimada Y, Kinugasa Y, Mori Y, et al. Site-specific tumor grading system in colorectal cancer: multicenter pathologic review of the value of quantifying poorly differentiated clusters. Am J Surg Pathol. 2014; 38:197–204.10. Barresi V, Bonetti LR, Ieni A, Branca G, Baron L, Tuccari G. Histologic grading based on counting poorly differentiated clusters in preoperative biopsy predicts nodal involvement and pTNM stage in colorectal cancer patients. Hum Pathol. 2014; 45:268–275.11. Barresi V, Branca G, Ieni A, Reggiani Bonetti L, Baron L, Mondello S, Tuccari G. Poorly differentiated clusters (PDCs) as a novel histological predictor of nodal metastases in pT1 colorectal cancer. Virchows Arch. 2014; 464:655–662.12. Young J, Simms LA, Biden KG, Wynter C, Whitehall V, Karamatic R, George J, Goldblatt J, Walpole I, Robin SA, et al. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol. 2001; 159:2107–2116.13. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Cancer Society. AJCC cancer staging handbook : from the AJCC cancer staging manual. 7th ed. New York: Springer;2010.14. Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989; 63:539–543.15. Lai YH, Wu LC, Li PS, Wu WH, Yang SB, Xia P, He XX, Xiao LB. Tumour budding is a reproducible index for risk stratification of patients with stage II colon cancer. Colorectal Dis. 2014; 16:259–264.16. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998; 58:5248–5257.17. Zlobec I, Bihl MP, Foerster A, Rufle A, Lugli A. The impact of CpG island methylator phenotype and microsatellite instability on tumour budding in colorectal cancer. Histopathology. 2012; 61:777–787.18. Jass JR, O'Brien MJ, Riddell RH, Snover DC. Association of Directors of Anatomic and Surgical Pathology (ADASP). Recommendations for the reporting of surgically resected specimens of colorectal carcinoma. Hum Pathol. 2007; 38:537–545.19. Jass JR, Barker M, Fraser L, Walsh MD, Whitehall VL, Gabrielli B, Young J, Leggett BA. APC mutation and tumour budding in colorectal cancer. J Clin Pathol. 2003; 56:69–73.20. Kim ST, Lee J, Park SH, Park JO, Lim HY, Kang WK, Kim JY, Kim YH, Chang DK, Rhee PL, et al. Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother Pharmacol. 2010; 66:659–667.21. Lin CC, Lin JK, Lin TC, Chen WS, Yang SH, Wang HS, Lan YT, Jiang JK, Yang MH, Chang SC. The prognostic role of microsatellite instability, codon-specific KRAS, and BRAF mutations in colon cancer. J Surg Oncol. 2014; 110:451–457.22. Chang SC, Lin JK, Yang SH, Wang HS, Li AF, Chi CW. Relationship between genetic alterations and prognosis in sporadic colorectal cancer. Int J Cancer. 2006; 118:1721–1727.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interactive computerized morphometric analysis for benign prostatic hyperplasia and prostatic adenocarcinoma
- Correlation MSI Status and Other Prognostic Factors in Sporadic Colorectal Cancer
- Clinicopathologic Features of Poorly Differentiated (Insular) Carcinoma of Thyroid
- Expression of HLA-DR antigen in large bowel carcinoma
- Clinicopathologic Feiatures and Indication of Endoscopic Treatment of Early Colorectal Cancer