J Korean Med Sci.
2014 Nov;29(Suppl 3):S176-S182. 10.3346/jkms.2014.29.S3.S176.
Treatment Algorithm of Complications after Filler Injection: Based on Wound Healing Process
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Kangnam Sacred Heart Hospital, Hallym University Medical Center, Seoul, Korea. sismdps@chol.com
- KMID: 2151411
- DOI: http://doi.org/10.3346/jkms.2014.29.S3.S176
Abstract
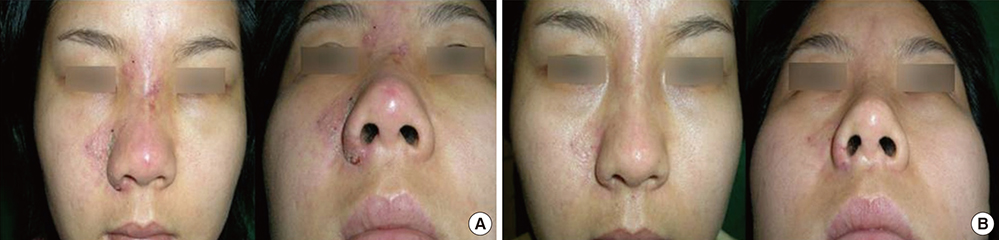
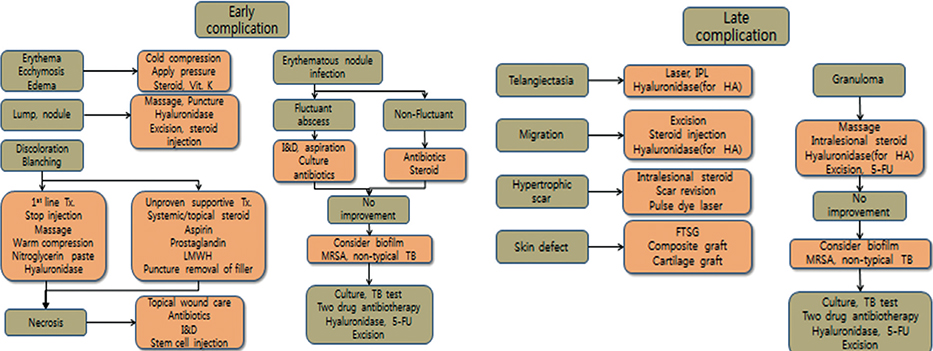
- Soft tissue filler injection has been a very common procedure worldwide since filler injection was first introduced for soft tissue augmentation. Currently, filler is used in various medical fields with satisfactory results, but the number of complications is increasing due to the increased use of filler. The complications after filler injection can occur at any time after the procedure, early and delayed, and they range from minor to severe. In this review, based on our experience and previously published other articles, we suggest a treatment algorithm to help wound healing and tissue regeneration and generate good aesthetic results with early treatment in response to the side effects of filler. Familiarity with the treatment of these rare complications is essential for achieving the best possible outcome.
MeSH Terms
Figure
Reference
-
1. Kim JE, Sykes JM. Hyaluronic acid fillers: history and overview. Facial Plast Surg. 2011; 27:523–528.2. Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006; 118:77s–84s.3. Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003; 27:354–366. discussion 67.4. Kinney BM, Hughes CE 3rd. Soft tissue fillers: an overview. Aesthet Surg J. 2001; 21:469–471.5. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013; 6:295–316.6. Vartanian AJ, Frankel AS, Rubin MG. Injected hyaluronidase reduces restylane-mediated cutaneous augmentation. Arch Facial Plast Surg. 2005; 7:231–237.7. Goldberg DJ. Legal ramifications of off-label filler use. Clin Plast Surg. 2006; 33:597–601.8. Cahill KV, Burns JA. Volume augmentation of the anophthalmic orbit with cross-linked collagen (Zyplast). Arch Ophthalmol. 1989; 107:1684–1686.9. Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000; 14:F25–F32.10. Chan RW, Titze IR. Viscosities of implantable biomaterials in vocal fold augmentation surgery. Laryngoscope. 1998; 108:725–731.11. Flint PW, Corio RL, Cummings CW. Comparison of soft tissue response in rabbits following laryngeal implantation with hydroxylapatite, silicone rubber, and Teflon. Ann Otol Rhinol Laryngol. 1997; 106:399–407.12. Hallen L, Johansson C, Laurent C. Cross-linked hyaluronan (Hylan B gel): a new injectable remedy for treatment of vocal fold insufficiency--an animal study. Acta Otolaryngol. 1999; 119:107–111.13. Dayan SH. Complications from toxins and fillers in the dermatology clinic: recognition, prevention, and treatment. Facial Plast Surg Clin North Am. 2013; 21:663–673.14. De Boulle K. Management of complications after implantation of fillers. J Cosmet Dermatol. 2004; 3:2–15.15. Jones D. Volumizing the face with soft tissue fillers. Clin Plast Surg. 2011; 38:379–390. v16. Rohrich RJ, Monheit G, Nguyen AT, Brown SA, Fagien S. Soft-tissue filler complications: the important role of biofilms. Plast Reconstr Surg. 2010; 125:1250–1256.17. Cohen JL, Bhatia AC. The role of topical vitamin K oxide gel in the resolution of postprocedural purpura. J Drugs Dermatol. 2009; 8:1020–1024.18. Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006; 118:92s–107s.19. Lemperle G, Duffy DM. Treatment options for dermal filler complications. Aesthet Surg J. 2006; 26:356–364.20. Alam M, Gladstone H, Kramer EM, Murphy JP Jr, Nouri K, Neuhaus IM, Spencer JM, Spenceri E, Van Dyke S, Ceilley RI, et al. ASDS guidelines of care: injectable fillers. Dermatol Surg. 2008; 34:S115–S148.21. Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg. 2009; 35:1672–1680.22. Alam M, Dover JS. Management of complications and sequelae with temporary injectable fillers. Plast Reconstr Surg. 2007; 120:98s–105s.23. Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008; 34:S92–S99.24. Graivier MH, Bass LS, Busso M, Jasin ME, Narins RS, Tzikas TL. Calcium hydroxylapatite (Radiesse) for correction of the mid- and lower face: consensus recommendations. Plast Reconstr Surg. 2007; 120:55s–66s.25. Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007; 6:846–847.26. Berlin A, Cohen JL, Goldberg DJ. Calcium hydroxylapatite for facial rejuvenation. Semin Cutan Med Surg. 2006; 25:132–137.27. Kang BS, Na YC, Jin YW. Comparison of the wound healing effect of cellulose and gelatin: an in vivo study. Arch Plast Surg. 2012; 39:317–321.28. Mioton LM, Jordan SW, Hanwright PJ, Bilimoria KY, Kim JY. The relationship between preoperative wound classification and postoperative infection: a multi-institutional analysis of 15,289 patients. Arch Plast Surg. 2013; 40:522–529.29. DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013; 33:561–575.30. Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature. J Cosmet Dermatol. 2011; 10:224–231.31. Kim SG, Kim YJ, Lee SI, Lee CJ. Salvage of nasal skin in a case of venous compromise after hyaluronic acid filler injection using prostaglandin E. Dermatol Surg. 2011; 37:1817–1819.32. Dayan SH, Arkins JP, Mathison CC. Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol. 2011; 10:1007–1012.33. Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013; 40:666–675.34. Sung HM, Suh IS, Lee HB, Tak KS, Moon KM, Jung MS. Case reports of adipose-derived stem cell therapy for nasal skin necrosis after filler injection. Arch Plast Surg. 2012; 39:51–54.35. Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012; 39:585–592.36. Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011; 31:110–121.37. Ko WJ, Na YC, Suh BS, Kim HA, Heo WH, Choi GH, Lee SU. The effects of topical agent (kelo-cote or contractubex) massage on the thickness of post-burn scar tissue formed in rats. Arch Plast Surg. 2013; 40:697–704.38. Carruthers A, Carruthers J. Non-animal-based hyaluronic acid fillers: scientific and technical considerations. Plast Reconstr Surg. 2007; 120:33s–40s.39. Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005; 31:893–897.40. Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. Treatment options. Plast Reconstr Surg. 2009; 123:1864–1873.41. Christensen LH. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol Surg. 2009; 35:1612–1619.42. Goldman MP. Pressure-induced migration of a permanent soft tissue filler. Dermatol Surg. 2009; 35:403–405. discussion 5-6.43. Pecharki D, Petersen FC, Scheie AA. Role of hyaluronidase in Streptococcus intermedius biofilm. Microbiology. 2008; 154:932–938.44. Bjarnsholt T, Tolker-Nielsen T, Givskov M, Janssen M, Christensen LH. Detection of bacteria by fluorescence in situ hybridization in culture-negative soft tissue filler lesions. Dermatol Surg. 2009; 35:1620–1624.45. Yoon TH, Yun IS, Rha DK, Lee WJ. Reconstruction of various perinasal defects using facial artery perforator-based nasolabial island flaps. Arch Plast Surg. 2013; 40:754–760.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adaptation of Evidence-based Surgical Wound Care Algorithm
- Nutritional Support and Wound Healing
- Case Reports of Adipose-derived Stem Cell Therapy for Nasal Skin Necrosis after Filler Injection
- The Clinical Uses of Collagen-Based Matrices in the Treatment of Chronic Wounds
- A Case of Platelet-rich Plasma (PRP) Treatment for Herpes Zoster Ophthalmicus-like Skin Necrosis after Filler Injection