Immune Netw.
2011 Jun;11(3):182-189. 10.4110/in.2011.11.3.182.
Enhanced Induction of T Cell Immunity Using Dendritic Cells Pulsed with HIV Tat and HCMV-pp65 Fusion Protein In Vitro
- Affiliations
-
- 1Catholic Hematopoietic Stem Cell Bank, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. kimth@catholic.ac.kr
- 2Department of Microbiology and Immunology, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2150706
- DOI: http://doi.org/10.4110/in.2011.11.3.182
Abstract
- BACKGROUND
Cytotoxic T lymphocytes (CTLs) appear to play an important role in the control and prevention of human cytomegalovirus (HCMV) infection. The pp65 antigen is a structural protein, which has been defined as a potential target for effective immunity against HCMV infection. Incorporation of an 11 amino acid region of the HIV TAT protein transduction domain (Tat) into protein facilitates rapid, efficient entry into cells.
METHODS
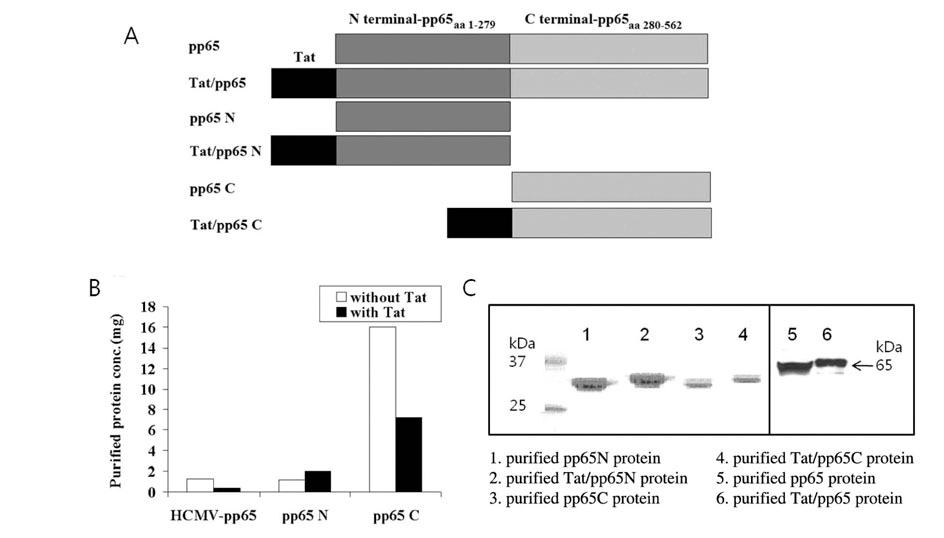
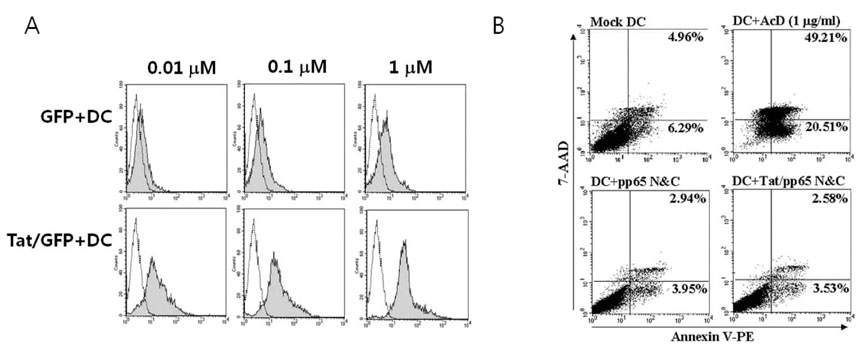
To establish a strategy for the generation of HCMV-specific CTLs in vitro, recombinant truncated N- and C-terminal pp65 protein (pp65 N&C) and N- and C-terminal pp65 protein fused with Tat (Tat/pp65 N&C) was produced in E.coli system. Peripheral blood mononuclear cells were stimulated with dendritic cells (DCs) pulsed with pp65 N&C or Tat/pp65 N&C protein and immune responses induced was examined using IFN-gamma ELISPOT assay, cytotoxicity assay and tetramer staining.
RESULTS
DCs pulsed with Tat/pp65N&C protein could induce higher T-cell responses in vitro compared with pp65N&C. Moreover, the DCs pulsed with Tat/pp65 N&C could stimulate both of CD8+ and CD4+ T-cell responses. The T cells induced by DCs pulsed with Tat/pp65 N&C showed higher cytotoxicity than that of pp65-pulsed DCs against autologous lymphoblastoid B-cell line (LCL) expressing the HCMV-pp65 antigen.
CONCLUSION
Our results suggest that DCs pulsed with Tat/pp65 N&C protein effectively induced pp65-specific CTL in vitro. Tat fusion recombinant protein may be useful for the development of adoptive T-cell immunotherapy and DC-based vaccines.
Keyword
MeSH Terms
Figure
Reference
-
1. Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990. 12:Suppl 7. S701–S710.
Article2. Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991. 72:2059–2064.
Article3. Minton EJ, Tysoe C, Sinclair JH, Sissons JG. Human cytomegalovirus infection of the monocytes/macrophages lineage in bone marrow. J Virol. 1994. 68:4017–4021.
Article4. Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997. 91:119–126.
Article5. Mendelson M, Monard S, Sissons P, Sinclair J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol. 1996. 77:3099–3102.
Article6. Michelson S. Interaction of human cytomegalovirus with monocytes/macrophages: a love-hate relationship. Pathol Biol (Paris). 1997. 45:146–158.7. Meyers JD, Flournoy N, Thomas ED. Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis. 1986. 153:478–488.
Article8. Meyers JD, Ljungman P, Fisher LD. Cytomegalovirus excretion as a predictor of cytomegalovirus disease after marrow transplantation: importance of cytomegalovirus viraemia. J Infect Dis. 1990. 162:373–380.
Article9. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245.
Article10. Foster AE, Forrester K, Gottlieb DJ, Barton GW, Romagnoli JA, Bradstock KF. Large-scale expansion of cytomegalovirus-specific cytotoxic T cells in suspension culture. Biotechnol Bioeng. 2004. 85:138–146.
Article11. Verma IM, Somia N. Gene therapy - promises, problems and prospects. Nature. 1997. 389:239–242.
Article12. Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevski SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998. 4:1449–1452.
Article13. Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999. 285:1569–1572.
Article14. Pande H, Baak SW, Riggs AD, Clark BR, Shively JE, Zaia JA. Cloning and physical mapping of a gene fragment coding for a 64-kilodalton major late antigen of human cytomegalovirus. Proc Natl Acad Sci USA. 1984. 81:4965–4969.
Article15. Kern F, Bunde T, Faulhaber N, Kiecker F, Khatamzas E, Rudawski IM, Pruss A, Gratama JW, Volkmer-Engert R, Ewert R, Reinke P, Volk HD, Picker LJ. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis. 2002. 185:1709–1716.
Article16. Bradford MA. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.
Article17. Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J Immunol. 1993. 151:3971–3980.18. Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, Sissons JG. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996. 70:7569–7579.
Article19. Cho HI, Han H, Kim CC, Kim TG. Generation of cytotoxic T lymphocytes specific for human cytomegalovirus using dendritic cells in vitro. J Immunother. 2001. 24:242–249.
Article20. Paston SJ, Dodi IA, Madrigal JA. Progress made towards the development of a CMV peptide vaccine. Human Immunology. 2004. 65:544–549.
Article21. Fittipaldi A, Giacca M. Transcellular protein transduction using the Tat protein of HIV-1. Adv Drug Deliv Rev. 2005. 57:597–608.
Article22. Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005. 57:579–596.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhanced Induction of CEA Specific Tumor Immunity by TatCEA Fusion Protein
- Enhanced CEA-specific Immune Responses by Tat-LLO Fusion Protein
- Human Immunodeficiency Virus Type 1 Tat-Mediated Cellular Response in Myeloid Cells
- Enhancement of Adenoviral Transduction and Immunogenecity of Transgenes by Soluble Coxsackie and Adenovirus Receptor-TAT Fusion Protein on Dendritic Cells
- p53-mediated Inhibitory Mechanism on HIV-1 Tat is Likely to be Associated with Tat-Phosphorylation