J Korean Med Assoc.
2006 Sep;49(9):790-798. 10.5124/jkma.2006.49.9.790.
Multidrug-resistant Tuberculosis
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine & Lung Institute, Seoul National University College of Medicine, Korea. yimjj@snu.ac.kr
- KMID: 2137819
- DOI: http://doi.org/10.5124/jkma.2006.49.9.790
Abstract
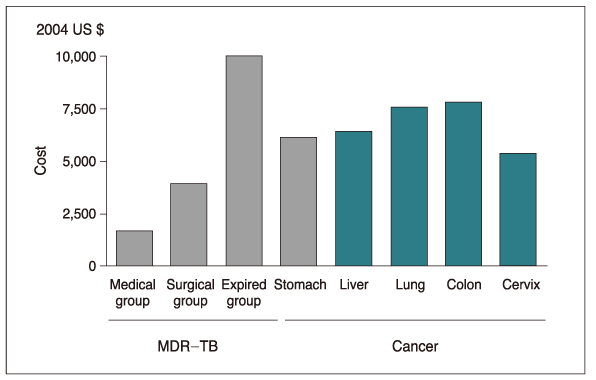
- Multidrug-resistant tuberculosis (MDR-TB), resistant to at least both isoniazid and rifampicin, poses a serious threat to global health because it requires treatment for a long duration and frequent hospitalization, and results in a considerable number of mortalities. According to a report from the World Health Organization in 2000, 3.2% of all new TB cases are MDR. In South Korea, multidrug-resistance was observed in 13% of re-treatment cases. The treatment of MDR-TB is difficult, since second-line drugs must be used, which are not as potent or as well tolerated as in the first-line drugs. Early publications on the treatment response of MDR-TB reported a considerable rate of mortality, as high as 37%. The use of 4 or 5 drugs including injectable drugs and fluoroquinolones is the fundamental of the medical treatment of MDR-TB. For patients with MDR-TB refractory to medical treatment, surgical resection could be tried. However, the candidate for the surgical resection should be selected cautiously. To overcome the low success rate of treatment among MDR-TB patients, well-designed clinical trials including newer drugs or regimens should be performed. MDR-TB has been a serious challenge to human health, especially in South Korea. To reduce the individual or social burden from MDR-TB, a commitment of government as well as clinicians is essential.
MeSH Terms
Figure
Reference
-
1. Dye C, Espinal MA, Watt CJ, Mbiaga C, Williams BG. Worldwide incidence of multidrug-resistant tuberculosis. J Infect Dis. 2002. 185:1197–1202.
Article2. Korean Center for Disease Control and prevention. Anti-tuberculosis durg resistance in Korea. Communicable Diseases Monthly Report. 2005. 16:101–107.3. Kim BJ, Lee IH, Lee DH, Bai GH, Kong SJ, Park SK, et al. The current status of multidrug-resistant tuberculosis in Korea. Tuber Lung Dis (In Korean). 2006. 60:404–411.
Article4. Mukherjee JS, Rich ML, Socci AR, Joseph JK, Viru FA, Seung KJ, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004. 363:474–481.
Article5. Kim HJ, Hong YP, Kim SJ, Lew WJ, Lee EG. Ambulatory treatment of multidrug-resistant pulmonary tuberculosis patients at a chest clinic. Int J Tuberc Lung Dis. 2001. 5:1129–1136.6. Gupta R, Kim JY, Espinal MA, Caudron JM, Pecoul B, Farmer PE, Raviglione MC. Public health. Responding to market failures in tuberculosis control. Science. 2001. 293:1049–1045.
Article7. Kang YA, Choi YJ, Lee SM, Yoo CG, Kim YW, Yim JJ, et al. Cost of treatment for multidrug-resistant tuberculosis in South Korea. Respirology. 2006. In press.
Article8. DOTS-PLUS and the Green Light Committee. WHO. http://www.who.int/tb/dots/dotsplus/management/en.9. WHO. WHO/CDC/TB.278. Anti-tuberculosis drug resistance in the world. Report No. 2: prevalence and trends. The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. 2000. Geneva: World Health Organization document.10. Korean Institute of Tuberculosis. Korean National Tuberculosis Association. Drug susceptibility testing on mycobacteria by the proportion method. Research report. 1994.11. Mitchison DA. What is drug resistance? Tubercle. 1969. 50:Suppl. 44–47.12. Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J. 2005. 25:564–569.
Article13. Caminero JA. Management of multidrug-resistant tuberculosis and patients in retreatment. Eur Respir J. 2005. 25:928–936.
Article14. WHO. Guidelines for the programmatic management of drug-resistant tuberculosis. 2006.15. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Vernon AA, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003. 167:603–662.
Article16. Maus CE, Plikaytis BB, Shinnick TM. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005. 49:3192–3197.
Article17. Sulochana S, Rahman F, Paramasivan CN. In vitro activity of fluoroquinolones against Mycobacterium tuberculosis. J Chemother. 2005. 17:169–173.18. Williams DL, Spring L, Collins L, Miller LP, Heifets LB, Gangadharam PR, Gillis TP. Contribution of rpoB mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998. 42:1853–1857.
Article19. Rastogi N, Labrousse V, Goh KS. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol. 1996. 33:167–175.
Article20. Pomerantz M, Madsen L, Goble M, Iseman M. Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg. 1991. 52:1108–1111. discussion 12.
Article21. Shiraishi Y, Nakajima Y, Katsuragi N, Kurai M, Takahashi N. Resectional surgery combined with chemotherapy remains the treatment of choice for multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg. 2004. 128:523–528.
Article22. Park SK, Lee CM, Heu JP, Song SD. A retrospective study for the outcome of pulmonary resection in 49 patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2002. 6:143–149.23. Pomerantz BJ, Cleveland JC Jr, Olson HK, Pomerantz M. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg. 2001. 121:448–453.
Article24. Chiang CY, Yu MC, Bai KJ, Suo J, Lin TP, Lee YC. Pulmonary resection in the treatment of patients with pulmonary multidrug-resistant tuberculosis in Taiwan. Int J Tuberc Lung Dis. 2001. 5:272–277.25. Sung SW, Kang CH, Kim YT, Han SK, Shim YS, Kim JH. Surgery increased the chance of cure in multi-drug resistant pulmonary tuberculosis. Eur J Cardiothorac Surg. 1999. 16:187–193.
Article26. van Leuven M, De Groot M, Shean KP, von Oppell UO, Willcox PA. Pulmonary resection as an adjunct in the treatment of multiple drug-resistant tuberculosis. Ann Thorac Surg. 1997. 63:1368–1372. discussion 72 - 3.
Article27. Kir A, Tahaoglu K, Okur E, Hatipoglu T. Role of surgery in multi-drug-resistant tuberculosis: results of 27 cases. Eur J Cardiothorac Surg. 1997. 12:531–534.
Article28. Treasure RL, Seaworth BJ. Current role of surgery in Mycobacterium tuberculosis. Ann Thorac Surg. 1995. 59:1405–1407. discussion 08 - 9.
Article29. Kim HJ, Kang CH, Kim YT, Sung SW, Kim JH, Yim JJ, et al. Prognostic factors for surgical resection in patients with multidrug-resistant tuberculosis. Eur Respir J. 2006. 28:563–567.
Article30. Kir A, Inci I, Torun T, Atasalihi A, Tahaoglu K. Adjuvant resectional surgery improves cure rates in multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg. 2006. 131:693–696.
Article31. Alcala L, Ruiz-Serrano MJ, Perez-Fernandez Turegano C, Garcia De Viedma D, Diaz-Infantes M, Marin-Arriaza M, Bouza E. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob Agents Chemother. 2003. 47:416–417.
Article32. Cynamon MH, Klemens SP, Sharpe CA, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1999. 43:1189–1191.
Article33. Fortun J, Martin-Davila P, Navas E, Perez-Elias MJ, Cobo J, Mereno S, et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother. 2005. 56:180–185.
Article34. von der Lippe B, Sandven P, Brubakk O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB)-a report of ten cases. J Infect. 2006. 52:92–96.
Article35. Park IN, Hong SB, Oh YM, Kim MN, Lim CM, Shim TS, et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J Antimicrob Chemother. 2006. 58(3):701–704.
Article36. Murray HW. Current and future clinical applications of interferon-gamma in host antimicrobial defense. Intensive Care Med. 1996. 22:Suppl 4. S456–S461.
Article37. Darnell JE Jr. Studies of IFN-induced transcriptional activation uncover the Jak-Stat pathway. J Interferon Cytokine Res. 1998. 18:549–554.
Article38. Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997. 349:1513–1515.
Article39. Suarez-Mendez R, Garcia-Garcia I, Fernandez-Olivera N, Valdes-Quintana M, Milanes-Mirelles MT, Lopez-Saura PA, et al. Adjuvant interferon gamma in patients with drug-resistant pulmonary tuberculosis: a pilot study. BMC Infect Dis. 2004. 4:44.40. Koh WJ, Kwon OJ, Suh GY, Chung MP, Kim H, Lee KS, et al. Six-month therapy with aerosolized interferon-gamma for refractory multidrug-resistant pulmonary tuberculosis. J Korean Med Sci. 2004. 19:167–171.
Article41. Kim EK, Shim TS, Lee JY, Oh YM, Lim CM, Kim WD, et al. The adjuvant effect of subcutaneous interferon-gamma in the treatment of refractory multidrug-resistant pulmonary tuberculosis. Tuber Lung Dis. 2004. 57:226–233. (In Korean).
Article42. Park SK, Chen DS, Lee IH, Lee DH. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma subcutaneous injection. Tuber Lung Dis. 2004. 55:S9. (In Korean).43. Stanford JL, Rook GA, Bahr GM, Dowlati Y, Genapati R, Minh Lv H, et al. Mycobacterium vaccae in immunoprophylaxis and immunotherapy of leprosy and tuberculosis. Vaccine. 1990. 8:525–530.
Article44. Luo Y, Lu S, Guo S. Immunotherapeutic effect of Mycobacterium vaccae on multi-drug resistant pulmonary tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi. 2000. 23:85–88.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multidrug-resistant Tuberculosis Spondylitis: A Case Report
- The survey for clinical course of intractable pulmonary tuberculosis
- Multidrug-resistant Tuberculosis
- Differentiating between Intestinal Tuberculosis and Crohn’s Disease May Be Complicated by Multidrug-resistant Mycobacterium tuberculosis
- Risk Factors for Primary Multidrug Resistant Tuberculosis