Korean J Hematol.
2007 Jun;42(2):106-113. 10.5045/kjh.2007.42.2.106.
The Role of Bone Marrow Mononuclear Cells in Angiogenesis in Mouse Hind Limb Ischemic Model
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. jakim@catholic.ac.kr
- 2Hematopoietic Stem Cell Transplantation Labaratory, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of General Surgery, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2083507
- DOI: http://doi.org/10.5045/kjh.2007.42.2.106
Abstract
-
BACKGROUND: Angiogenesis is enhanced in the ischemic tissues after the injection of bone marrow cells (BMCs). However the exact mechanisms for this are not yet fully understood.
METHODS
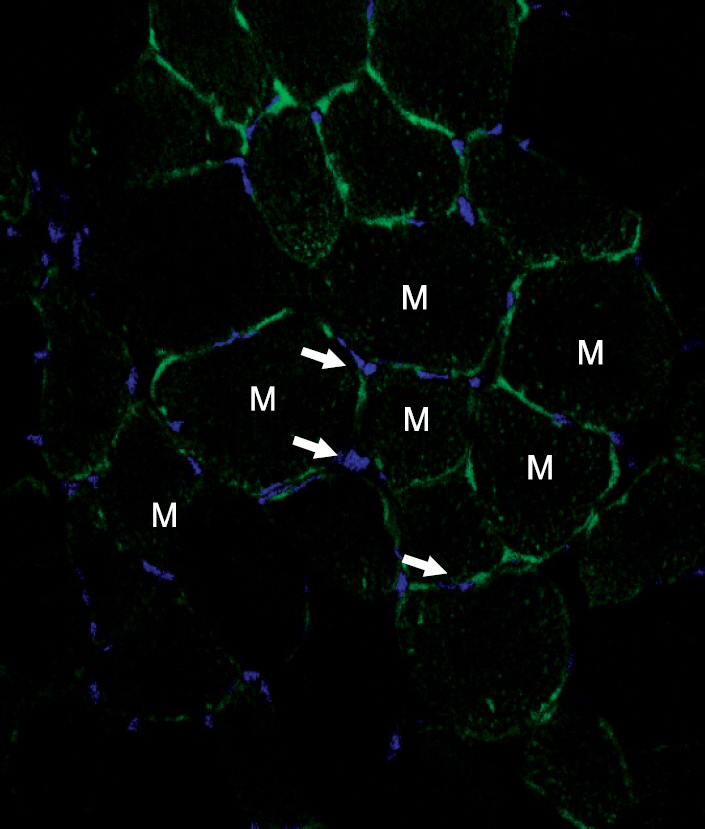
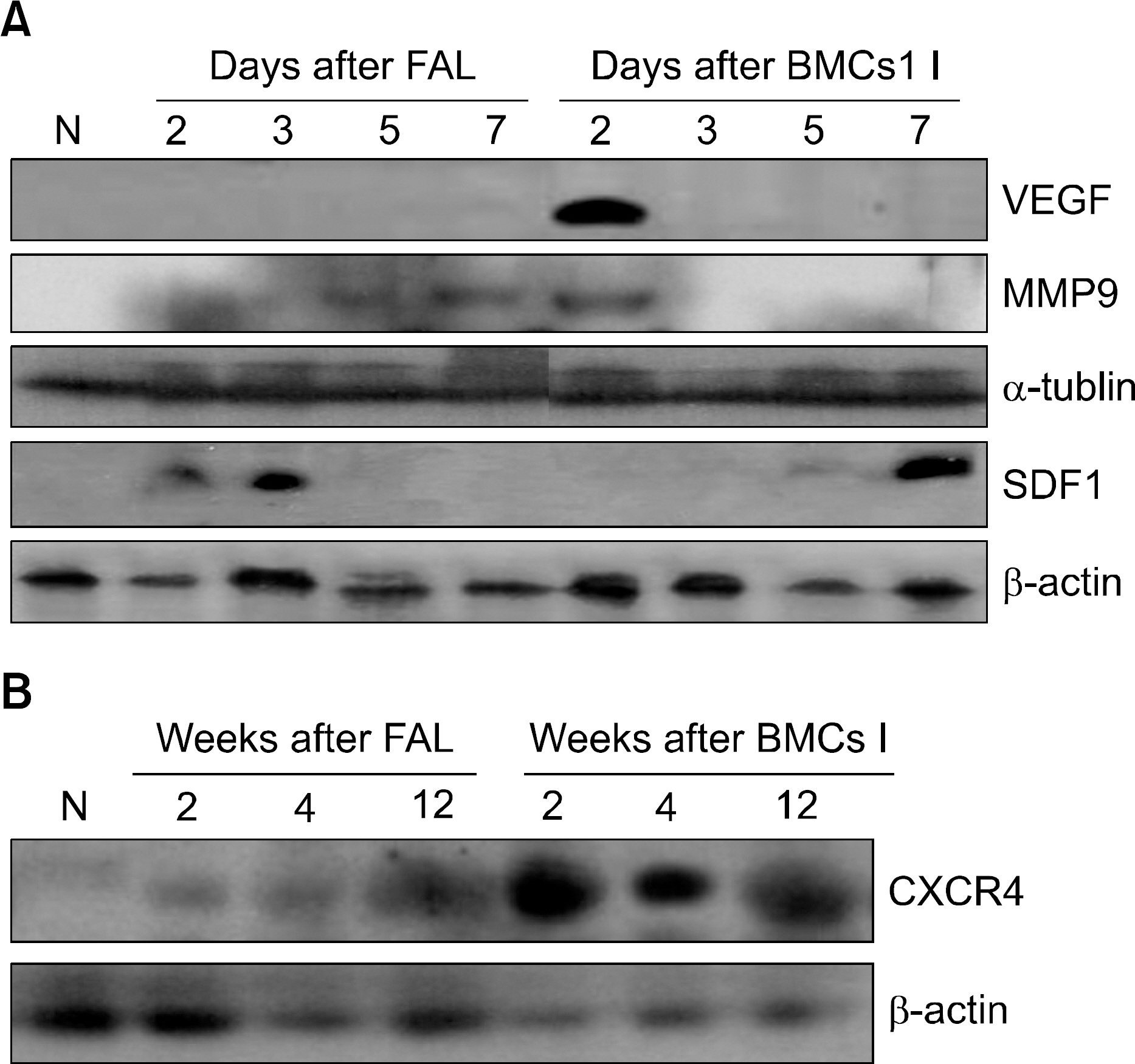
A unilateral ischemic limb was surgically induced in mice and then BMCs were injected into the ischemic area. We measured the capillary/muscle ratio. Fluorescence-labeled BMCs were injected into the ischemic tissues and then the locations of the cells were examined by using a confocal microscope. Recruitment of bone marrow-derived cells into the ischemic tissue was examined in a sex-mismatched bone marrow transplantation (BMT) setting by identifying the Y chromosome with using the FISH technique. The expressions of VEGF, MMP-9, SDF-1 and CXCR-4 were measured by Western blot analysis.
RESULTS
The capillary/muscle ratio was more increased in the BMC-injected group than in the control group (P<0.05). Florescence-labeled BMCs, which had been directly injected into ischemic tissue, were not detected in the tissue. In the sex-mismatched bone marrow transplantation models, the ischemic tissues of the BMC-injected group recruited a much greater number of Y chromosome-positive bone marrow- derived cells, as compared to the control group. The expressions of VEGF and MMP-9 were increased after injection of BMCs. SDF-1 was expressed on the seventh day in the BMC-injected group and CXCR-4 was highly expressed until 12 weeks in the BMC-injected group.
CONCLUSION
We suggest that the injection of BMCs into ischemic tissue recruits CXCR-4-positvie cells from the bone marrow via the up-regulation of VEGF, MMP-9 and SDF-1, and these CXCR-4-positive cells may play a role in neovascularization.
Keyword
MeSH Terms
Figure
Reference
-
1). Grunewald M., Avraham I., Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006. 124:175–89.
Article2). Zentilin L., Tafuro S., Zacchigna S, et al. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006. 107:3546–54.
Article3). Ruiz de Almodovar C., Luttun A., Carmeliet P. An SDF-1 trap for myeloid cells stimulates angiogenesis. Cell. 2006. 124:18–21.
Article4). Jin DK., Shido K., Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006. 12:557–67.
Article5). Abbott JD., Huang Y., Liu D., Hickey R., Krause DS., Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004. 110:3300–5.6). Peled A., Petit I., Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999. 283:845–8.
Article7). Sipkins DA., Wei X., Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdoma-ins for tumour engraftment. Nature. 2005. 435:969–73.
Article8). Shintani S., Murohara T., Ikeda H, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001. 103:897–903.
Article9). Heissig B., Hattori K., Dias S, et al. Recruitment of stem and progenitor cells from th bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002. 109:625–37.10). Asahara T., Masuda H., Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–8.
Article11). Ribatti D. The involvement of endothelial progenitor cells in tumor angiogenesis. J Cell Mol Med. 2004. 8:294–300.
Article12). Gunsilius E., Duba HC., Petzer AL, et al. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000. 355:1688–91.
Article13). Fuchs S., Kornowski R., Weisz G, et al. Safety and feasibility of transendocardial autologous bone marrow cell transplantation in patients with advanced heart disease. Am J Cardiol. 2006. 97:823–9.
Article14). Tse HF., Kwong YL., Chan JK., Lo G., Ho CL., Lau CP. Angiogenesis in ischemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003. 361:47–9.15). Rafii S., Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003. 9:702–12.
Article16). Luttun A., Tjwa M., Moons L, et al. Revascularization of ischemic tissues by PIGF treatment, and inhibition of tumor angiogenesis, arthritis and artherosclerosis by anti-Flt1. Nat Med. 2002. 8:831–40.17). Colson YL., Wren SM., Schuchert MJ, et al. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995. 155:4179–88.18). Jin DK., Shido K., Kopp HG., Petit I, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006. 12:557–67.
Article19). O'Neill TJ., Wamhoff BR., Owens GK., Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without trans-differentiation into endothelial cells. Circ Res. 2005. 97:1027–35.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Angiogenesis Induced by the Implantation of Autogenous Whole Bone Marrow Stem Cells in an Ischemic Animal Model
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- Therapeutic Angiogenesis for Cardiovascular Diseases: The Present and Future
- A Study for Correlation between Bone Marrow Micrometastases and Tumoric Angiogenesis in Breast Cancer
- The Study of BD-MSC Therapy against Critical Limb Ischemia