Yonsei Med J.
2015 Mar;56(2):388-396. 10.3349/ymj.2015.56.2.388.
Clinical Experience of Glioma Surgery Using "Tailed Bullet": Overcoming the Limitations of Conventional Neuro-Navigation Guided Surgery
- Affiliations
-
- 1Department of Neurosurgery, Catholic Kwandong University, International St. Mary's Hospital, Incheon, Korea.
- 2Department of Neurosurgery, Bundang CHA Medical Center, CHA University College of Medicine, Seongnam, Korea. sandori50@gmail.com
- 3Department of Neurosurgery, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2070015
- DOI: http://doi.org/10.3349/ymj.2015.56.2.388
Abstract
- PURPOSE
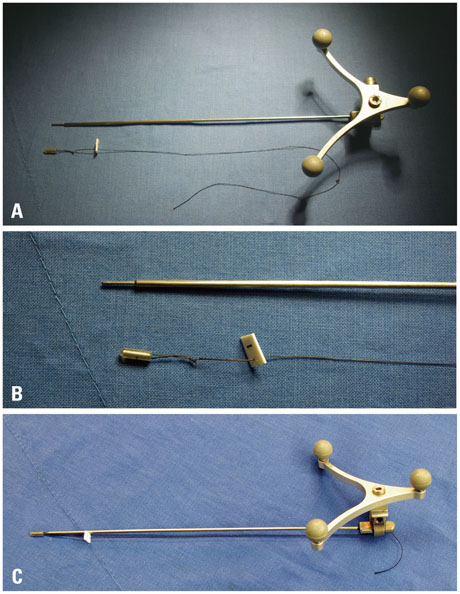
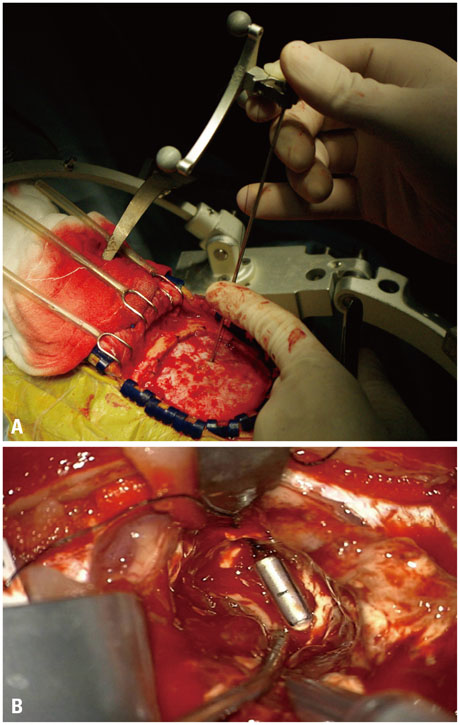
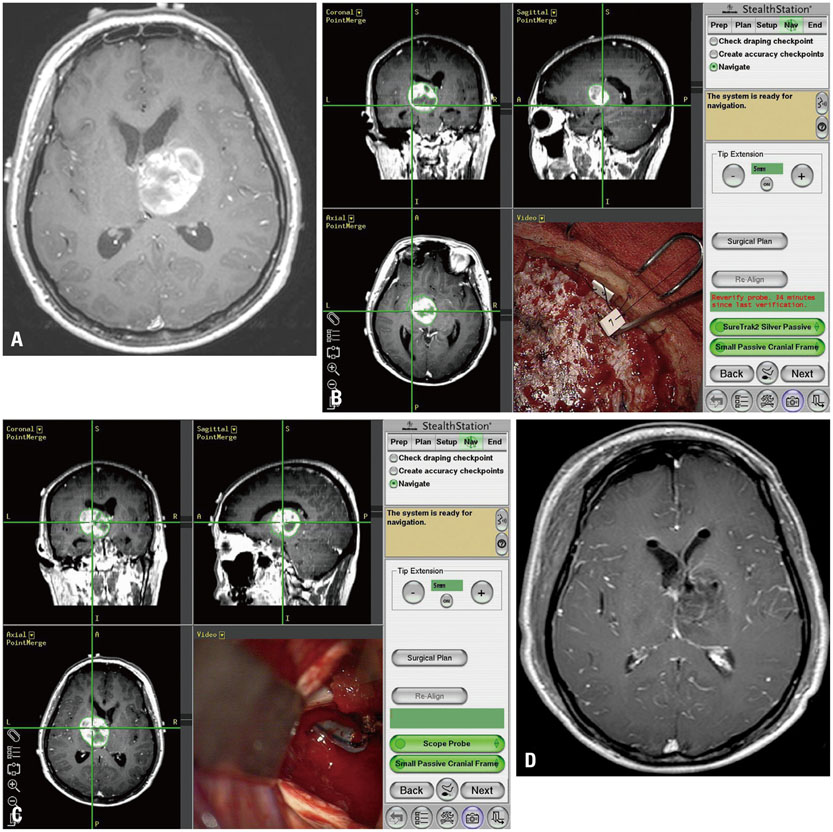
Although conventional neuro-navigation is a useful tool for image-guided glioma surgery, there are some limitations, such as brain shift. We introduced our methods using an identifiable marker, a "tailed bullet", to overcome the limitation of conventional neuro-navigation. A tailed bullet is an identifiable tumor location marker that determines the extent of a resection and we have introduced our technique and reviewed the clinical results.
MATERIALS AND METHODS
We have developed and used "tailed bullets" for brain tumor surgery. They were inserted into the brain parenchyma or the tumor itself to help identify the margin of tumor. We retrospectively reviewed surgically resected glioma cases using "tailed bullet". Total 110 gliomas included in this study and it contains WHO grade 2, 3, and 4 glioma was 14, 36, and 60 cases, respectively.
RESULTS
Gross total resection (GTR) was achieved in 71 patients (64.5%), subtotal resection in 36 patients (32.7%), and partial resection in 3 patients (2.7%). The overall survival (OS) duration of grade 3 and 4 gliomas were 20.9 (range, 1.2-82.4) and 13.6 months (range, 1.4-173.4), respectively. Extent of resection (GTR), younger age, and higher initial Karnofsky Performance Status (KPS) score were related to longer OS for grade-4 gliomas. There was no significant adverse event directly related to the use of tailed bullets.
CONCLUSION
Considering the limitations of conventional neuro-navigation methods, the tailed bullets could be helpful during glioma resection. We believe this simple method is an easily accessible technique and overcomes the limitation of the brain shift from the conventional neuro-navigation. Further studies are needed to verify the clinical benefits of using tailed bullets.
Keyword
MeSH Terms
-
Adult
Aged
Brain/pathology
Brain Neoplasms/pathology/*surgery
Female
Glioma/pathology/*surgery
Humans
Karnofsky Performance Status
Magnetic Resonance Imaging, Interventional
Male
Middle Aged
Neuronavigation/*methods
Retrospective Studies
Surgery, Computer-Assisted/*methods
Survival Rate
Time Factors
Treatment Outcome
Figure
Reference
-
1. Chaichana KL, Martinez-Gutierrez JC, De la Garza-Ramos R, Weingart JD, Olivi A, Gallia GL, et al. Factors associated with survival for patients with glioblastoma with poor pre-operative functional status. J Clin Neurosci. 2013; 20:818–823.
Article2. Moliterno JA, Patel TR, Piepmeier JM. Neurosurgical approach. Cancer J. 2012; 18:20–25.
Article3. Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003; 99:467–473.
Article4. Stummer W, Meinel T, Ewelt C, Martus P, Jakobs O, Felsberg J, et al. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neurooncol. 2012; 108:89–97.
Article5. Duffau H. Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir (Wien). 2012; 154:575–584.
Article6. McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009; 110:156–162.
Article7. Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien). 2011; 153:1211–1218.
Article8. Pouratian N, Asthagiri A, Jagannathan J, Shaffrey ME, Schiff D. Surgery Insight: the role of surgery in the management of low-grade gliomas. Nat Clin Pract Neurol. 2007; 3:628–639.
Article9. Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012; 308:1881–1888.
Article10. Ius T, Isola M, Budai R, Pauletto G, Tomasino B, Fadiga L, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012; 117:1039–1052.
Article11. Bianco Ade M, Miura FK, Clara C, Almeida JR, Silva CC, Teixeira MJ, et al. Low-grade astrocytoma: surgical outcomes in eloquent versus non-eloquent brain areas. Arq Neuropsiquiatr. 2013; 71:31–34.
Article12. Makary M, Chiocca EA, Erminy N, Antor M, Bergese SD, Abdel-Rasoul M, et al. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. J Magn Reson Imaging. 2011; 34:1022–1030.
Article13. Cho KG, Ahn YH. Stereotactic resection of the brain tumor using 'tailed bullets': technical note. J Korean Neurosurg Soc. 1998; 27:619–624.14. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.
Article15. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006; 7:392–401.
Article16. Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000; 93:1003–1013.
Article17. Uhl E, Zausinger S, Morhard D, Heigl T, Scheder B, Rachinger W, et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery. 2009; 64:5 Suppl 2. 231–239.
Article18. Nimsky C, Ganslandt O, Hastreiter P, Fahlbusch R. Intraoperative compensation for brain shift. Surg Neurol. 2001; 56:357–364.
Article19. Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000; 47:1070–1079.
Article20. Nabavi A, Black PM, Gering DT, Westin CF, Mehta V, Pergolizzi RS Jr, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001; 48:787–797.
Article21. Reinges MH, Nguyen HH, Krings T, Hütter BO, Rohde V, Gilsbach JM. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien). 2004; 146:369–377.
Article22. Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011; 12:997–1003.
Article23. Rutigliano MJ. Cost effectiveness analysis: a review. Neurosurgery. 1995; 37:436–443.24. Ramina R, Coelho Neto M, Giacomelli A, Barros E Jr, Vosgerau R, Nascimento A, et al. Optimizing costs of intraoperative magnetic resonance imaging. A series of 29 glioma cases. Acta Neurochir (Wien). 2010; 152:27–33.
Article25. Tsugu A, Ishizaka H, Mizokami Y, Osada T, Baba T, Yoshiyama M, et al. Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg. 2011; 76:120–127.
Article26. Sherman JH, Hoes K, Marcus J, Komotar RJ, Brennan CW, Gutin PH. Neurosurgery for brain tumors: update on recent technical advances. Curr Neurol Neurosci Rep. 2011; 11:313–319.
Article27. Utsuki S, Miyoshi N, Oka H, Miyajima Y, Shimizu S, Suzuki S, et al. Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol. 2007; 24:53–55.
Article28. Utsuki S, Oka H, Sato S, Shimizu S, Suzuki S, Tanizaki Y, et al. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo). 2007; 47:210–213.
Article29. Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011; 115:740–748.
Article30. Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994; 74:1784–1791.
Article31. Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008; 26:1338–1345.
Article32. Hirschberg H, Samset E, Hol PK, Tillung T, Lote K. Impact of intraoperative MRI on the surgical results for high-grade gliomas. Minim Invasive Neurosurg. 2005; 48:77–84.
Article33. Jolesz FA. Future perspectives for intraoperative MRI. Neurosurg Clin N Am. 2005; 16:201–213.
Article34. Kelly PJ, Kall BA, Goerss S, Earnest F 4th. Computer-assisted stereotaxic laser resection of intra-axial brain neoplasms. J Neurosurg. 1986; 64:427–439.
Article35. Yoshikawa K, Kajiwara K, Morioka J, Fujii M, Tanaka N, Fujisawa H, et al. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol. 2006; 78:91–97.
Article36. Wong JM, Panchmatia JR, Ziewacz JE, Bader AM, Dunn IF, Laws ER, et al. Patterns in neurosurgical adverse events: intracranial neoplasm surgery. Neurosurg Focus. 2012; 33:E16.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stereotactic Resection of the Brain Tumor Using 'Tailed Bullets': Technical Note
- Comparative Analysis of Anterior Cervical Discectomy and Fusion at a Single Level: Free-Hand Versus Navigation-Guided Approaches
- Accuracy and Safety in Pedicle Screw Placement in the Thoracic and Lumbar Spines : Comparison Study between Conventional C-Arm Fluoroscopy and Navigation Coupled with O-Arm(R) Guided Methods
- Navigation Guided Open Wedge High Tibial Osteotomy
- Spinal surgery using image-guided navigation systems: a technical note