Korean J Radiol.
2015 Feb;16(1):99-113. 10.3348/kjr.2015.16.1.99.
Imaging Findings of Common Benign Renal Tumors in the Era of Small Renal Masses: Differential Diagnosis from Small Renal Cell Carcinoma: Current Status and Future Perspectives
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul 110-744, Korea. radjycho@snu.ac.kr
- 2Institute of Radiation Medicine and Kidney Research Institute, Seoul National University Medical Research Center, Seoul 110-744, Korea.
- KMID: 2069988
- DOI: http://doi.org/10.3348/kjr.2015.16.1.99
Abstract
- The prevalence of small renal masses (SRM) has risen, paralleling the increased usage of cross-sectional imaging. A large proportion of these SRMs are not malignant, and do not require invasive treatment such as nephrectomy. Therefore, differentation between early renal cell carcinoma (RCC) and benign SRM is critical to achieve proper management. This article reviews the radiological features of benign SRMs, with focus on two of the most common benign entities, angiomyolipoma and oncocytoma, in terms of their common imaging findings and differential features from RCC. Furthermore, the role of percutaneous biopsy is discussed as imaging is yet imperfect, therefore necessitating biopsy in certain circumstances to confirm the benignity of SRMs.
MeSH Terms
-
Abdominal Fat/pathology
Adenoma, Oxyphilic/diagnosis/radiography/ultrasonography
Angiomyolipoma/diagnosis/radiography/ultrasonography
Carcinoma, Renal Cell/*diagnosis/radiography/ultrasonography
Diagnosis, Differential
Humans
Kidney Neoplasms/*diagnosis/*radiography/ultrasonography
Leiomyoma/diagnosis/radiography/ultrasonography
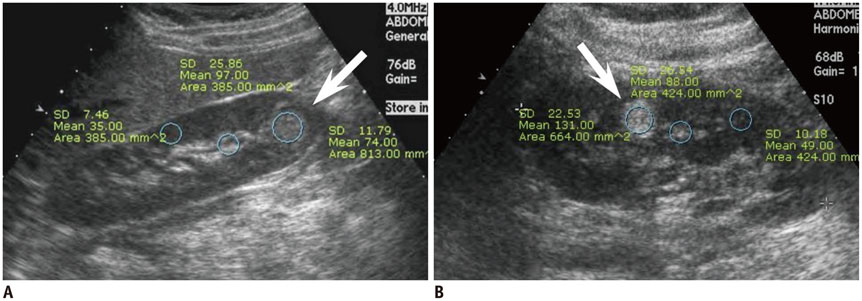
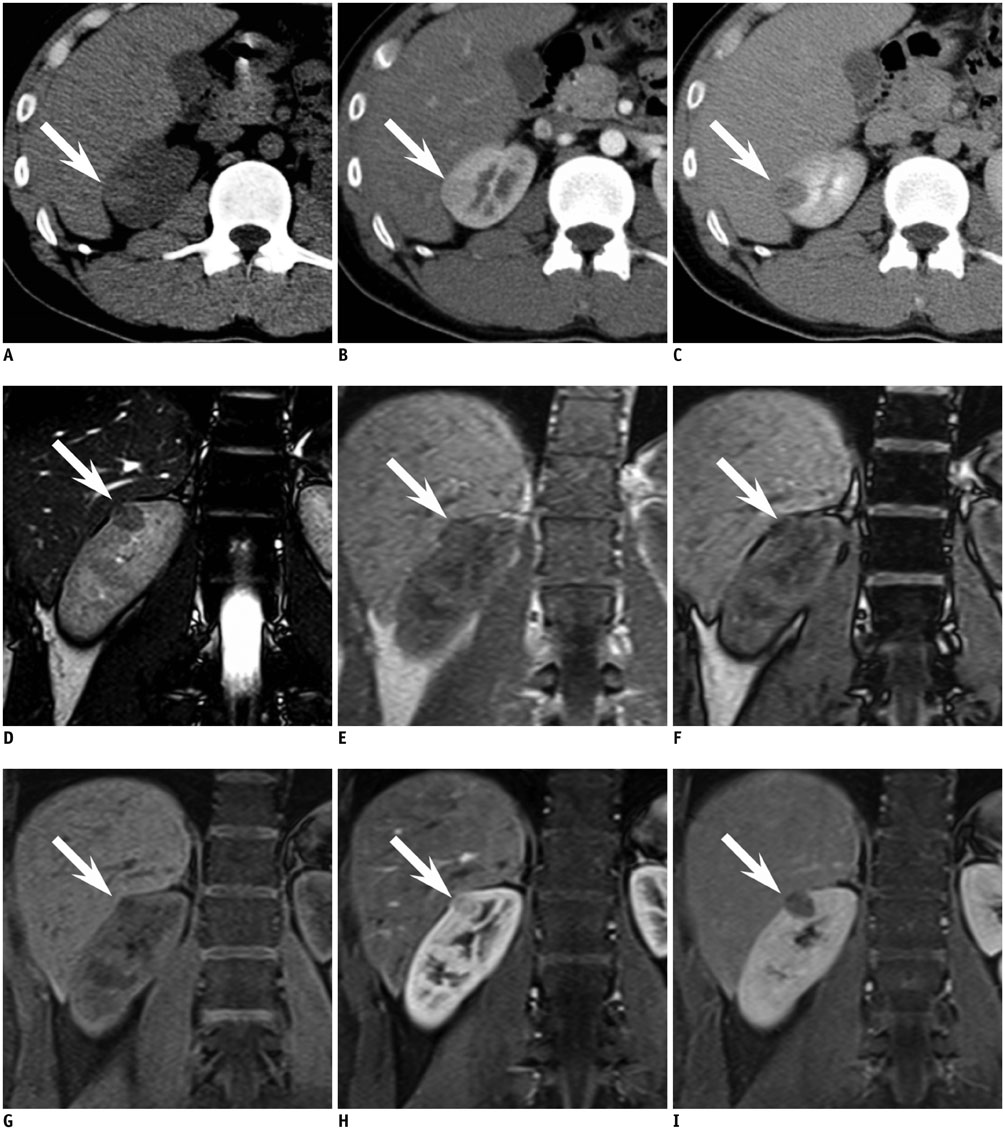
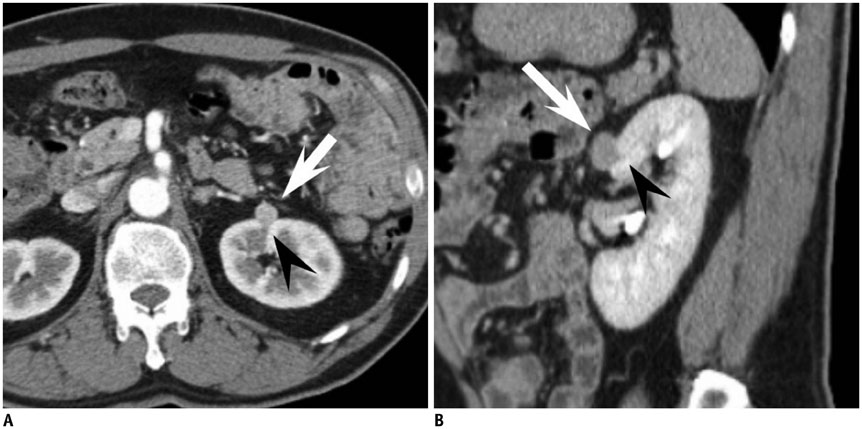
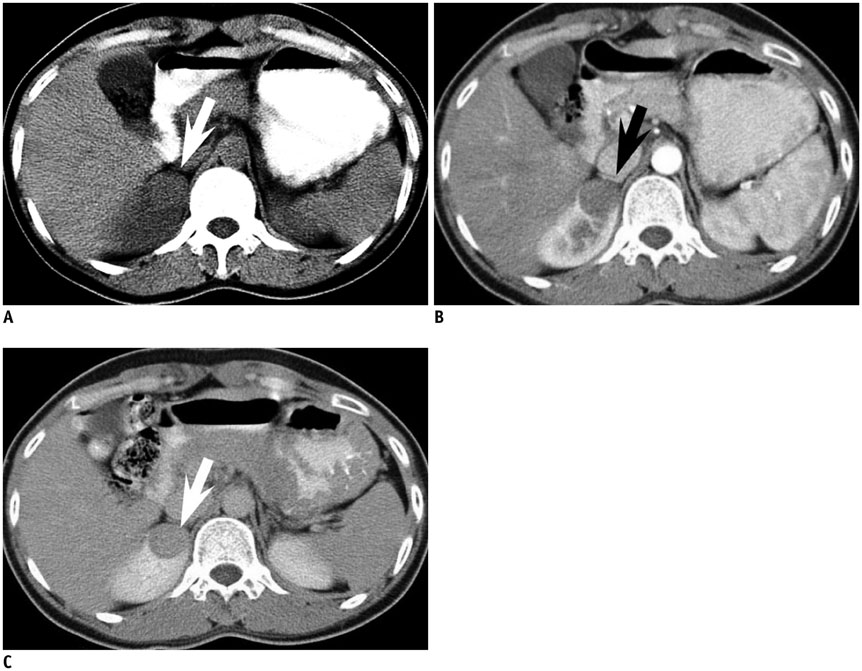
Figure
Cited by 1 articles
-
Gastrointestinal Involvement of Recurrent Renal Cell Carcinoma: CT Findings and Clinicopathologic Features
Hyo Jung Park, Hyun Jin Kim, Seong Ho Park, Jong Seok Lee, Ah Young Kim, Hyun Kwon Ha
Korean J Radiol. 2017;18(3):452-460. doi: 10.3348/kjr.2017.18.3.452.
Reference
-
1. Silverman SG, Israel GM, Herts BR, Richie JP. Management of the incidental renal mass. Radiology. 2008; 249:16–31.2. Gill IS, Aron M, Gervais DA, Jewett MA. Clinical practice. Small renal mass. N Engl J Med. 2010; 362:624–634.3. Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010; 7:245–257.4. Reis LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al. SEER Cancer Statistics Review, 1975-2007. Bethesda, MD: National Cancer Institute;2008.5. Rioux-Leclercq N, Karakiewicz PI, Trinh QD, Ficarra V, Cindolo L, de la Taille A, et al. Prognostic ability of simplified nuclear grading of renal cell carcinoma. Cancer. 2007; 109:868–874.6. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003; 170(6 Pt 1):2217–2220.7. Thompson RH, Kurta JM, Kaag M, Tickoo SK, Kundu S, Katz D, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol. 2009; 181:2033–2036.8. Sivalingam S, Nakada SY. Contemporary minimally invasive treatment options for renal angiomyolipomas. Curr Urol Rep. 2013; 14:147–153.9. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization Classification of Tumors. Pathology and genetics of tumors of the Urinary System and Male Genital Organs. Lyon: IARC Press;2004. p. 65–66.10. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000; 7:47–66.11. Lane BR, Aydin H, Danforth TL, Zhou M, Remer EM, Novick AC, et al. Clinical correlates of renal angiomyolipoma subtypes in 209 patients: classic, fat poor, tuberous sclerosis associated and epithelioid. J Urol. 2008; 180:836–843.12. Siegel CL, Middleton WD, Teefey SA, McClennan BL. Angiomyolipoma and renal cell carcinoma: US differentiation. Radiology. 1996; 198:789–793.13. Jinzaki M, Ohkuma K, Tanimoto A, Mukai M, Hiramatsu K, Murai M, et al. Small solid renal lesions: usefulness of power Doppler US. Radiology. 1998; 209:543–550.14. Bosniak MA, Megibow AJ, Hulnick DH, Horii S, Raghavendra BN. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR Am J Roentgenol. 1988; 151:497–501.15. Lemaitre L, Claudon M, Dubrulle F, Mazeman E. Imaging of angiomyolipomas. Semin Ultrasound CT MR. 1997; 18:100–114.16. Israel GM, Hindman N, Hecht E, Krinsky G. The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipomas. AJR Am J Roentgenol. 2005; 184:1868–1872.17. Takahashi K, Honda M, Okubo RS, Hyodo H, Takakusaki H, Yokoyama H, et al. CT pixel mapping in the diagnosis of small angiomyolipomas of the kidneys. J Comput Assist Tomogr. 1993; 17:98–101.18. Kurosaki Y, Tanaka Y, Kuramoto K, Itai Y. Improved CT fat detection in small kidney angiomyolipomas using thin sections and single voxel measurements. J Comput Assist Tomogr. 1993; 17:745–748.19. Hafron J, Fogarty JD, Hoenig DM, Li M, Berkenblit R, Ghavamian R. Imaging characteristics of minimal fat renal angiomyolipoma with histologic correlations. Urology. 2005; 66:1155–1159.20. Jinzaki M, Tanimoto A, Narimatsu Y, Ohkuma K, Kurata T, Shinmoto H, et al. Angiomyolipoma: imaging findings in lesions with minimal fat. Radiology. 1997; 205:497–502.21. Trigaux JP, Pauls C, Van Beers B. Atypical renal hamartomas: ultrasonography, computed tomography, and angiographic findings. J Clin Ultrasound. 1993; 21:41–44.22. Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014; 39:588–604.23. Lee MS, Cho JY, Kim SH. Ultrasonographic differentiation of small angiomyolipoma from renal cell carcinoma by measuring relative echogenicity on PACS. J Korean Soc Ultrasound Med. 2010; 29:105–113.24. Kim JK, Park SY, Shon JH, Cho KS. Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology. 2004; 230:677–684.25. Woo S, Cho JY, Kim SH, Kim SY. Angiomyolipoma with minimal fat and non-clear cell renal cell carcinoma: differentiation on MDCT using classification and regression tree analysis-based algorithm. Acta Radiol. 2014; 55:1258–1269.26. Yang CW, Shen SH, Chang YH, Chung HJ, Wang JH, Lin AT, et al. Are there useful CT features to differentiate renal cell carcinoma from lipid-poor renal angiomyolipoma? AJR Am J Roentgenol. 2013; 201:1017–1028.27. Kim MH, Lee J, Cho G, Cho KS, Kim J, Kim JK. MDCT-based scoring system for differentiating angiomyolipoma with minimal fat from renal cell carcinoma. Acta Radiol. 2013; 54:1201–1209.28. Simpfendorfer C, Herts BR, Motta-Ramirez GA, Lockwood DS, Zhou M, Leiber M, et al. Angiomyolipoma with minimal fat on MDCT: can counts of negative-attenuation pixels aid diagnosis? AJR Am J Roentgenol. 2009; 192:438–443.29. Chaudhry HS, Davenport MS, Nieman CM, Ho LM, Neville AM. Histogram analysis of small solid renal masses: differentiating minimal fat angiomyolipoma from renal cell carcinoma. AJR Am J Roentgenol. 2012; 198:377–383.30. Catalano OA, Samir AE, Sahani DV, Hahn PF. Pixel distribution analysis: can it be used to distinguish clear cell carcinomas from angiomyolipomas with minimal fat? Radiology. 2008; 247:738–746.31. Choi HJ, Kim JK, Ahn H, Kim CS, Kim MH, Cho KS. Value of T2-weighted MR imaging in differentiating low-fat renal angiomyolipomas from other renal tumors. Acta Radiol. 2011; 52:349–353.32. Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A. Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology. 2012; 263:160–168.33. Chung MS, Choi HJ, Kim MH, Cho KS. Comparison of T2-weighted MRI with and without fat suppression for differentiating renal angiomyolipomas without visible fat from other renal tumors. AJR Am J Roentgenol. 2014; 202:765–771.34. Kido T, Yamashita Y, Sumi S, Baba Y, Takahashi M, Ootsuka Y, et al. Chemical shift GRE MRI of renal angiomyolipoma. J Comput Assist Tomogr. 1997; 21:268–270.35. Kim JK, Kim SH, Jang YJ, Ahn H, Kim CS, Park H, et al. Renal angiomyolipoma with minimal fat: differentiation from other neoplasms at double-echo chemical shift FLASH MR imaging. Radiology. 2006; 239:174–180.36. Hindman N, Ngo L, Genega EM, Melamed J, Wei J, Braza JM, et al. Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology. 2012; 265:468–477.37. Oliva MR, Glickman JN, Zou KH, Teo SY, Mortelé KJ, Rocha MS, et al. Renal cell carcinoma: t1 and t2 signal intensity characteristics of papillary and clear cell types correlated with pathology. AJR Am J Roentgenol. 2009; 192:1524–1530.38. Verma SK, Mitchell DG, Yang R, Roth CG, O'Kane P, Verma M, et al. Exophytic renal masses: angular interface with renal parenchyma for distinguishing benign from malignant lesions at MR imaging. Radiology. 2010; 255:501–507.39. Kim KH, Yun BH, Jung SI, Hwang IS, Hwang EC, Kang TW, et al. Usefulness of the ice-cream cone pattern in computed tomography for prediction of angiomyolipoma in patients with a small renal mass. Korean J Urol. 2013; 54:504–509.40. van den Berg E, Dijkhuizen T, Oosterhuis JW, Geurts van, de Jong B, Störkel S. Cytogenetic classification of renal cell cancer. Cancer Genet Cytogenet. 1997; 95:103–107.41. Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS. Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J Surg Pathol. 1997; 21:621–635.42. Tan S, Özcan MF, Tezcan F, Balci S, Karaoğlanoğlu M, Huddam B, et al. Real-time elastography for distinguishing angiomyolipoma from renal cell carcinoma: preliminary observations. AJR Am J Roentgenol. 2013; 200:W369–W375.43. Tanaka H, Yoshida S, Fujii Y, Ishii C, Tanaka H, Koga F, et al. Diffusion-weighted magnetic resonance imaging in the differentiation of angiomyolipoma with minimal fat from clear cell renal cell carcinoma. Int J Urol. 2011; 18:727–730.44. Sasamori H, Saiki M, Suyama J, Ohgiya Y, Hirose M, Gokan T. Utility of apparent diffusion coefficients in the evaluation of solid renal tumors at 3T. Magn Reson Med Sci. 2014; 13:89–95.45. Agnello F, Roy C, Bazille G, Galia M, Midiri M, Charles T, et al. Small solid renal masses: characterization by diffusion-weighted MRI at 3 T. Clin Radiol. 2013; 68:e301–e308.46. Störkel S, Pannen B, Thoenes W, Steart PV, Wagner S, Drenckhahn D. Intercalated cells as a probable source for the development of renal oncocytoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988; 56:185–189.47. Geramizadeh B, Ravanshad M, Rahsaz M. Useful markers for differential diagnosis of oncocytoma, chromophobe renal cell carcinoma and conventional renal cell carcinoma. Indian J Pathol Microbiol. 2008; 51:167–171.48. Kurup AN, Thompson RH, Leibovich BC, Harmsen WS, Sebo TJ, Callstrom MR, et al. Renal oncocytoma growth rates before intervention. BJU Int. 2012; 110:1444–1448.49. Goiney RC, Goldenberg L, Cooperberg PL, Charboneau JW, Rosenfield AT, Russin LD, et al. Renal oncocytoma: sonographic analysis of 14 cases. AJR Am J Roentgenol. 1984; 143:1001–1004.50. Charboneau JW, Hattery RR, Ernst EC 3rd, James EM, Williamson B Jr, Hartman GW. Spectrum of sonographic findings in 125 renal masses other than benign simple cyst. AJR Am J Roentgenol. 1983; 140:87–94.51. Quinn MJ, Hartman DS, Friedman AC, Sherman JL, Lautin EM, Pyatt RS, et al. Renal oncocytoma: new observations. Radiology. 1984; 153:49–53.52. Jasinski RW, Amendola MA, Glazer GM, Bree RL, Gikas PW. Computed tomography of renal oncocytomas. Comput Radiol. 1985; 9:307–314.53. Ambos MA, Bosniak MA, Valensi QJ, Madayag MA, Lefleur RS. Angiographic patterns in renal oncocytomas. Radiology. 1978; 129:615–622.54. Davidson AJ, Hayes WS, Hartman DS, McCarthy WF, Davis CJ Jr. Renal oncocytoma and carcinoma: failure of differentiation with CT. Radiology. 1993; 186:693–696.55. Kim JI, Cho JY, Moon KC, Lee HJ, Kim SH. Segmental enhancement inversion at biphasic multidetector CT: characteristic finding of small renal oncocytoma. Radiology. 2009; 252:441–448.56. Woo S, Cho JY, Kim SH, Kim SY, Lee HJ, Hwang SI, et al. Segmental enhancement inversion of small renal oncocytoma: differences in prevalence according to tumor size. AJR Am J Roentgenol. 2013; 200:1054–1059.57. Woo S, Cho JY, Kim SH, Kim SY. Comparison of segmental enhancement inversion on biphasic MDCT between small renal oncocytomas and chromophobe renal cell carcinomas. AJR Am J Roentgenol. 2013; 201:598–604.58. McGahan JP, Lamba R, Fisher J, Starshak P, Ramsamooj R, Fitzgerald E, et al. Is segmental enhancement inversion on enhanced biphasic MDCT a reliable sign for the noninvasive diagnosis of renal oncocytomas? AJR Am J Roentgenol. 2011; 197:W674–W679.59. O'Malley ME, Tran P, Hanbidge A, Rogalla P. Small renal oncocytomas: is segmental enhancement inversion a characteristic finding at biphasic MDCT? AJR Am J Roentgenol. 2012; 199:1312–1315.60. Schieda N, McInnes MD, Cao L. Diagnostic accuracy of segmental enhancement inversion for diagnosis of renal oncocytoma at biphasic contrast enhanced CT: systematic review. Eur Radiol. 2014; 24:1421–1429.61. Alshumrani G, O'Malley M, Ghai S, Metser U, Kachura J, Finelli A, et al. Small (< or = 4 cm) cortical renal tumors: characterization with multidetector CT. Abdom Imaging. 2010; 35:488–493.62. Gakis G, Kramer U, Schilling D, Kruck S, Stenzl A, Schlemmer HP. Small renal oncocytomas: differentiation with multiphase CT. Eur J Radiol. 2011; 80:274–278.63. Bird VG, Kanagarajah P, Morillo G, Caruso DJ, Ayyathurai R, Leveillee R, et al. Differentiation of oncocytoma and renal cell carcinoma in small renal masses (<4 cm): the role of 4-phase computerized tomography. World J Urol. 2011; 29:787–792.64. Rosenkrantz AB, Hindman N, Fitzgerald EF, Niver BE, Melamed J, Babb JS. MRI features of renal oncocytoma and chromophobe renal cell carcinoma. AJR Am J Roentgenol. 2010; 195:W421–W427.65. Cornelis F, Lasserre AS, Tourdias T, Deminière C, Ferrière JM, Le Bras Y, et al. Combined late gadolinium-enhanced and double-echo chemical-shift MRI help to differentiate renal oncocytomas with high central T2 signal intensity from renal cell carcinomas. AJR Am J Roentgenol. 2013; 200:830–838.66. Cornelis F, Tricaud E, Lasserre AS, Petitpierre F, Bernhard JC, Le Bras Y, et al. Routinely performed multiparametric magnetic resonance imaging helps to differentiate common subtypes of renal tumours. Eur Radiol. 2014; 24:1068–1080.67. Lanzman RS, Robson PM, Sun MR, Patel AD, Mentore K, Wagner AA, et al. Arterial spin-labeling MR imaging of renal masses: correlation with histopathologic findings. Radiology. 2012; 265:799–808.68. Lassel EA, Rao R, Schwenke C, Schoenberg SO, Michaely HJ. Diffusion-weighted imaging of focal renal lesions: a meta-analysis. Eur Radiol. 2014; 24:241–249.69. Choyke PL. Imaging of hereditary renal cancer. Radiol Clin North Am. 2003; 41:1037–1051.70. Rowsell C, Fleshner N, Marrano P, Squire J, Evans A. Papillary renal cell carcinoma within a renal oncocytoma: case report of an incidental finding of a tumour within a tumour. J Clin Pathol. 2007; 60:426–428.71. Davis CJ Jr, Barton JH, Sesterhenn IA, Mostofi FK. Metanephric adenoma. Clinicopathological study of fifty patients. Am J Surg Pathol. 1995; 19:1101–1114.72. Patankar T, Punekar S, Madiwale C, Prasad S, Hanchate V. Metanephric adenoma in a solitary kidney. Br J Radiol. 1999; 72:80–81.73. Fielding JR, Visweswaran A, Silverman SG, Granter SR, Renshaw AA. CT and ultrasound features of metanephric adenoma in adults with pathologic correlation. J Comput Assist Tomogr. 1999; 23:441–444.74. Araki T, Hata H, Asakawa E, Araki T. MRI of metanephric adenoma. J Comput Assist Tomogr. 1998; 22:87–90.75. Prasad SR, Surabhi VR, Menias CO, Raut AA, Chintapalli KN. Benign renal neoplasms in adults: cross-sectional imaging findings. AJR Am J Roentgenol. 2008; 190:158–164.76. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010; 30:1525–1540.77. Steiner M, Quinlan D, Goldman SM, Millmond S, Hallowell MJ, Stutzman RE, et al. Leiomyoma of the kidney: presentation of 4 new cases and the role of computerized tomography. J Urol. 1990; 143:994–998.78. Radvany MG, Shanley DJ, Gagliardi JA. Magnetic resonance imaging with computed tomography of a renal leiomyoma. Abdom Imaging. 1994; 19:67–69.79. Martin SA, Mynderse LA, Lager DJ, Cheville JC. Juxtaglomerular cell tumor: a clinicopathologic study of four cases and review of the literature. Am J Clin Pathol. 2001; 116:854–863.80. Conn JW, Cohen EL, Lucas CP, McDonald WJ, Mayor GH, Blough WM Jr, et al. Primary reninism. Hypertension, hyperreninemia, and secondary aldosteronism due to renin-producing juxtaglomerular cell tumors. Arch Intern Med. 1972; 130:682–696.81. Prasad SR, Narra VR, Shah R, Humphrey PA, Jagirdar J, Catena JR, et al. Segmental disorders of the nephron: histopathological and imaging perspective. Br J Radiol. 2007; 80:593–602.82. Dunnick NR, Hartman DS, Ford KK, Davis CJ Jr, Amis ES Jr. The radiology of juxtaglomerular tumors. Radiology. 1983; 147:321–326.83. Remzi M, Katzenbeisser D, Waldert M, Klingler HC, Susani M, Memarsadeghi M, et al. Renal tumour size measured radiologically before surgery is an unreliable variable for predicting histopathological features: benign tumours are not necessarily small. BJU Int. 2007; 99:1002–1006.84. Duchene DA, Lotan Y, Cadeddu JA, Sagalowsky AI, Koeneman KS. Histopathology of surgically managed renal tumors: analysis of a contemporary series. Urology. 2003; 62:827–830.85. Silver DA, Morash C, Brenner P, Campbell S, Russo P. Pathologic findings at the time of nephrectomy for renal mass. Ann Surg Oncol. 1997; 4:570–574.86. Herts BR, Baker ME. The current role of percutaneous biopsy in the evaluation of renal masses. Semin Urol Oncol. 1995; 13:254–261.87. Rybicki FJ, Shu KM, Cibas ES, Fielding JR, vanSonnenberg E, Silverman SG. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003; 180:1281–1287.88. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009; 9:44–55.89. Helm CW, Burwood RJ, Harrison NW, Melcher DH. Aspiration cytology of solid renal tumours. Br J Urol. 1983; 55:249–253.90. Murphy WM, Zambroni BR, Emerson LD, Moinuddin S, Lee LH. Aspiration biopsy of the kidney. Simultaneous collection of cytologic and histologic specimens. Cancer. 1985; 56:200–205.91. Bonzanini M, Pea M, Martignoni G, Zamboni G, Capelli P, Bernardello F, et al. Preoperative diagnosis of renal angiomyolipoma: fine needle aspiration cytology and immunocytochemical characterization. Pathology. 1994; 26:170–175.92. Gupta RK, Nowitz M, Wakefield SJ. Fine-needle aspiration cytology of renal angiomyolipoma: report of a case with immunocytochemical and electron microscopic findings. Diagn Cytopathol. 1998; 18:297–300.93. Liu J, Fanning CV. Can renal oncocytomas be distinguished from renal cell carcinoma on fine-needle aspiration specimens? A study of conventional smears in conjunction with ancillary studies. Cancer. 2001; 93:390–397.94. Renshaw AA, Lee KR, Madge R, Granter SR. Accuracy of fine needle aspiration in distinguishing subtypes of renal cell carcinoma. Acta Cytol. 1997; 41:987–994.95. Zhou M, Roma A, Magi-Galluzzi C. The usefulness of immunohistochemical markers in the differential diagnosis of renal neoplasms. Clin Lab Med. 2005; 25:247–257.96. Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991; 178:253–258.97. Silverman SG, Gan YU, Mortele KJ, Tuncali K, Cibas ES. Renal masses in the adult patient: the role of percutaneous biopsy. Radiology. 2006; 240:6–22.98. Wehle MJ, Grabstald H. Contraindications to needle aspiration of a solid renal mass: tumor dissemination by renal needle aspiration. J Urol. 1986; 136:446–448.99. Slywotzky C, Maya M. Needle tract seeding of transitional cell carcinoma following fine-needle aspiration of a renal mass. Abdom Imaging. 1994; 19:174–176.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidental Solid Renal Masses: Radiologic Assessment and Managements
- Percutaneous Renal Tumor Biopsy in Small Renal Masses
- Imaging-Guided Biopsy, Percutaneous Ablation, and Active Surveillance for Small Renal Masses
- The Comparison between Pre- and Postoperative Diagnosis in Renal Masses Smaller than 3cm in Diameter
- Small Renal Masses: Surgery or Surveillance