Anat Cell Biol.
2013 Mar;46(1):39-48. 10.5115/acb.2013.46.1.39.
Distribution of elastic fibers in the head and neck: a histological study using late-stage human fetuses
- Affiliations
-
- 1Department of Anatomy, Tokyo Dental College, Chiba, Japan. abesh@tdc.ac.jp
- 2Department of Anatomy and Embryology II, Faculty of Medicine, Complutense University, Madrid, Spain.
- 3Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- KMID: 2046756
- DOI: http://doi.org/10.5115/acb.2013.46.1.39
Abstract
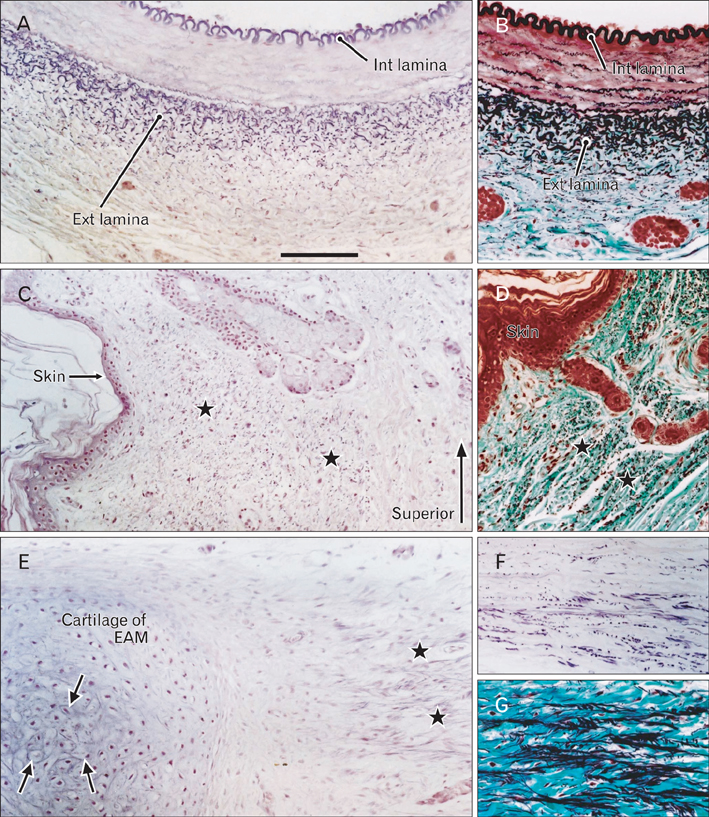
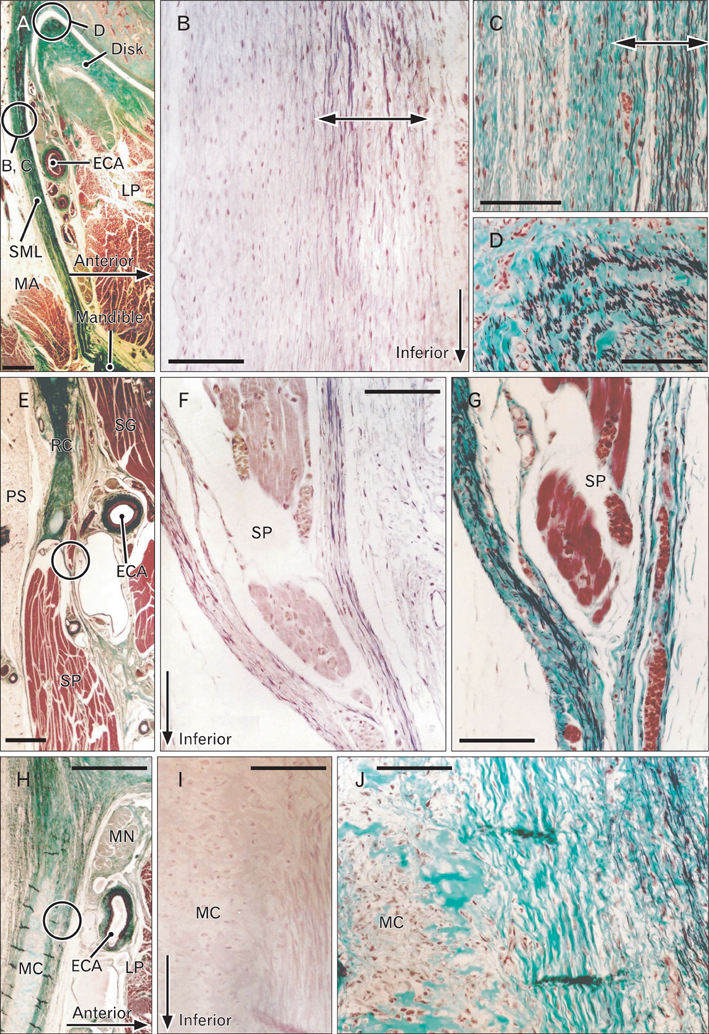
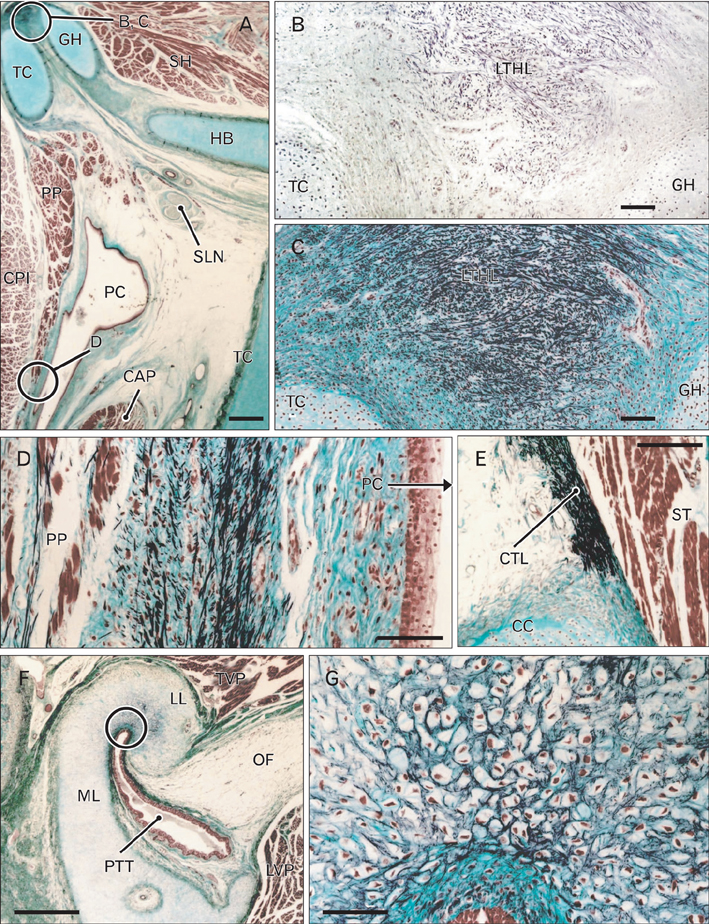
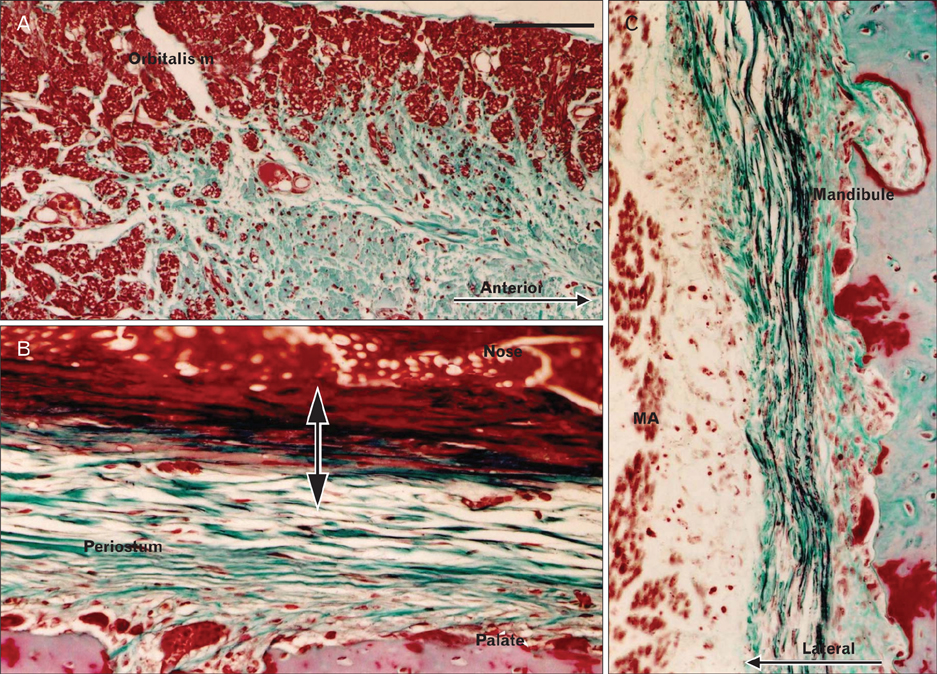
- There is little or no information about the distribution of elastic fibers in the human fetal head. We examined this issue in 15 late-stage fetuses (crown-rump length, 220-320 mm) using aldehyde-fuchsin and elastica-Masson staining, and we used the arterial wall elastic laminae and external ear cartilages as positive staining controls. The posterior pharyngeal wall, as well as the ligaments connecting the laryngeal cartilages, contained abundant elastic fibers. In contrast with the sphenomandibular ligament and the temporomandibular joint disk, in which elastic fibers were partly present, the discomalleolar ligament and the fascial structures around the pterygoid muscles did not have any elastic fibers. In addition, the posterior marginal fascia of the prestyloid space did contain such fibers. Notably, in the middle ear, elastic fibers accumulated along the tendons of the tensor tympani and stapedius muscles and in the joint capsules of the ear ossicle articulations. Elastic fibers were not seen in any other muscle tendons or vertebral facet capsules in the head and neck. Despite being composed of smooth muscle, the orbitalis muscle did not contain any elastic fibers. The elastic fibers in the sphenomandibular ligament seemed to correspond to an intermediate step of development between Meckel's cartilage and the final ligament. Overall, there seemed to be a mini-version of elastic fiber distribution compared to that in adults and a different specific developmental pattern of connective tissues. The latter morphology might be a result of an adaptation to hypoxic conditions during development.
MeSH Terms
Figure
Cited by 1 articles
-
Fetal cervical zygapophysial joint with special reference to the associated synovial tissue: a histological study using near-term human fetuses
Kei Kitamura, Shogo Hayashi, Zhe Wu Jin, Masahito Yamamoto, Gen Murakami, José Francisco Rodríguez-Vázquez, Hitoshi Yamamoto
Anat Cell Biol. 2021;54(1):65-73. doi: 10.5115/acb.20.265.
Reference
-
1. Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000. 258:1–14.2. Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002. 43:2923–2932.3. Osanai H, Murakami G, Ohtsuka A, Suzuki D, Nakagawa T, Tatsumi H. Histotopographical study of human periocular elastic fibers using aldehyde-fuchsin staining with special reference to the sleeve and pulley system for extraocular rectus muscles. Anat Sci Int. 2009. 84:129–140.4. Osanai H, Abe S, Rodríguez-Vázquez J, Verdugo-López S, Murakami G, Ohguro H. Human orbital muscle: a new point of view from the fetal development of extraocular connective tissues. Invest Ophthalmol Vis Sci. 2011. 52:1501–1506.5. Fukuda Y, Ferrans VJ, Crystal RG. Development of elastic fibers of nuchal ligament, aorta, and lung of fetal and post natal sheep: an ultrastructural and electron microscopic immunohistochemical study. Am J Anat. 1984. 170:597–629.6. Nakamura T, Hashimoto N, Maeda Y, Ikeda T, Nakagawa H, Takagi K. Degeneration and ossification of the yellow ligament in unstable spine. J Spinal Disord. 1990. 3:288–292.7. Viejo-Fuertes D, Liguoro D, Rivel J, Midy D, Guerin J. Morphologic and histologic study of the ligamentum flavum in the thoraco-lumbar region. Surg Radiol Anat. 1998. 20:171–176.8. Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and aging. Microsc Res Tech. 1997. 38:428–435.9. Shiraishi Y, Kobayashi M, Yasui M, Ozaki N, Sugiura Y. Innervation and functional characteristics of connective tissues, especially elastic fibers, in human fetal thoracic intervertebral articular capsule and its surroundings. Anat Embryol (Berl). 2003. 206:437–445.10. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, Kudo Y. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011. 24:469–477.11. Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, Usui T. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008. 19:1663–1670.12. Kinugasa Y, Arakawa T, Abe S, Ohtsuka A, Suzuki D, Murakami G, Fujimiya M, Sugihara K. Anatomical reevaluation of the anococcygeal ligament and its surgical relevance. Dis Colon Rectum. 2011. 54:232–237.13. Nagata I, Murakami G, Suzuki D, Furuya K, Koyama M, Ohtsuka A. Histological features of the rectovaginal septum in elderly women and a proposal for posterior vaginal defect repair. Int Urogynecol J Pelvic Floor Dysfunct. 2007. 18:863–868.14. Nagy NB, Daniel JC. Distribution of elastic fibres in the developing rabbit craniomandibular joint. Arch Oral Biol. 1991. 36:15–23.15. O'Dell NL, Starcher BC, Wilson JT, Pennington CB, Jones GA. Morphological and biochemical evidence for elastic fibres in the Syrian hamster temporomandibular joint disc. Arch Oral Biol. 1990. 35:807–811.16. Ueki S, Iwai-Liao Y, Tsubai T, Kojima H, Higashi Y. Fine structure of cartilage elastic system fibers, in particular those of the mandibular condyle. J Osaka Dent Univ. 1989. 23:99–109.17. Caltabiano M, Caltabiano C, Martinez G, Leonardi R. Histology of the cartilage of the human fetal condyle. Mondo Ortod. 1990. 15:439–442.18. Baccarani-Contri M, Taparelli F, Pasquali-Ronchetti I. Osteopontin is a constitutive component of normal elastic fibers in human skin and aorta. Matrix Biol. 1995. 14:553–560.19. Perrotta I, Russo E, Camastra C, Filice G, Di Mizio G, Colosimo F, Ricci P, Tripepi S, Amorosi A, Triumbari F, Donato G. New evidence for a critical role of elastin in calcification of native heart valves: immunohistochemical and ultrastructural study with literature review. Histopathology. 2011. 59:504–513.20. Harada Y, Ishizeki K. Evidence for transformation of chondrocytes and site-specific resorption during the degradation of Meckel's cartilage. Anat Embryol (Berl). 1998. 197:439–450.21. Richany SF, Bast TH, Anson BJ. The development of the first branchial arch in man and the fate of Meckel's cartilage. Q Bull Northwest Univ Med Sch. 1956. 30:331–355.22. Rodríguez Vázquez JF, Merida Velasco JR, Jimenez Collado J. Development of the human sphenomandibular ligament. Anat Rec. 1992. 233:453–460.23. Rodríguez-Vázquez JF. Development of the stapes and associated structures in human embryos. J Anat. 2005. 207:165–173.24. Rodriguez-Vazquez JF, Mérida-Velasco JR, Verdugo-López S, Sánchez-Montesinos I, Mérida-Velasco JA. Morphogenesis of the second pharyngeal arch cartilage (Reichert's cartilage) in human embryos. J Anat. 2006. 208:179–189.25. Rodríguez-Vázquez JF. Development of the stapedius muscle and pyramidal eminence in humans. J Anat. 2009. 215:292–299.26. Fujita T. Histological studies on the neuro-insular complex in the pancreas of some mammals. Z Zellforsch. 1959. 50:94–109.27. Sunami-Kataoka Y, Akagi H, Nishizaki K, Taguchi T, Murakami T, Ohtsuka A. Chondroitin sulfate proteoglycan at the basal lamina beneath high endothelial cells in human palatine tonsils: a light and electron microscopic study using the cationic colloidal iron method. Arch Histol Cytol. 2001. 64:535–543.28. Hayashi T, Kumasaka T, Mitani K, Yao T, Suda K, Seyama K. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010. 23:1251–1260.29. Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, Yoshimoto T. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemical study. Acta Neurochir (Wien). 1995. 136:88–91.30. Okeda R, Arima K, Kawai M. Arterial changes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in relation to pathogenesis of diffuse myelin loss of cerebral white matter: examination of cerebral medullary arteries by reconstruction of serial sections of an autopsy case. Stroke. 2002. 33:2565–2569.31. Chong VF, Mukherji SK, Goh CH. The suprahyoid neck: normal and pathological anatomy. J Laryngol Otol. 1999. 113:501–508.32. Li QY, Zhang SX, Liu ZJ, Tan LW, Qiu MG, Li K, Cui GY, Guo YL, Yang XP, Zhang WG, Chen XH, Chen JH, Ding SY, Chen W, You J, Wang YS, Deng JH, Tang ZS. The pre-styloid compartment of the parapharyngeal space: a three-dimensional digitized model based on the Chinese Visible Human. Surg Radiol Anat. 2004. 26:411–416.33. Christov A, Korol RM, Dai E, Liu L, Guan H, Bernards MA, Cavers PB, Susko D, Lucas A. In vivo optical analysis of quantitative changes in collagen and elastin during arterial remodeling. Photochem Photobiol. 2005. 81:457–466.34. Zheng Q, Choi J, Rouleau L, Leask RL, Richardson JA, Davis EC, Yanagisawa H. Normal wound healing in mice deficient for fibulin-5, an elastin binding protein essential for dermal elastic fiber assembly. J Invest Dermatol. 2006. 126:2707–2714.35. Shin JH, Lee HK, Kim SY, Choi CG, Suh DC. Imaging of parapharyngeal space lesions: focus on the prestyloid compartment. AJR Am J Roentgenol. 2001. 177:1465–1470.36. Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000. 10:312–320.37. Ritty TM, Ditsios K, Starcher BC. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat Rec. 2002. 268:430–440.38. Korol RM, Finlay HM, Josseau MJ, Lucas AR, Canham PB. Fluorescence spectroscopy and birefringence of molecular changes in maturing rat tail tendon. J Biomed Opt. 2007. 12:024011.39. Gleason PD, Beall DP, Sanders TG, Bond JL, Ly JQ, Holland LL, Pasque CB. The transverse humeral ligament: a separate anatomical structure or a continuation of the osseous attachment of the rotator cuff? Am J Sports Med. 2006. 34:72–77.40. Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007. 62:375–381.41. Stecco C, Porzionato A, Macchi V, Tiengo C, Parenti A, Aldegheri R, Delmas V, De Caro R. Histological characteristics of the deep fascia of the upper limb. Ital J Anat Embryol. 2006. 111:105–110.42. Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002. 187:93–98.43. Hirata E, Koyama M, Murakami G, Ohtsuka A, Abe S, Ide Y, Fujiwara H, Kudo Y. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011. 37:13–23.44. Guadall A, Orriols M, Rodríguez-Calvo R, Calvayrac O, Crespo J, Aledo R, Martínez-Gonzalez J, Rodríguez C. Fibulin-5 is upregulated by hypoxia in endothelial cells through a hypoxia-inducible factor-1 (HIF-1alpha)-dependent mechanism. J Biol Chem. 2011. 286:7093–7103.45. Cotta-Pereira G, Guerra Rodrigo F, Bittencourt-Sampaio S. Oxytalan, elaunin, and elastic fibers in the human skin. J Invest Dermatol. 1976. 66:143–148.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on the Development of Lung and Distribution of Elastic and Reticular Fibers during Fetal Period Proper
- The Morphologic Study of Elastic Structures in the Developing Murine Eustachian Tube
- Generalized Pseudoxanthoma Elasticum
- Histopathologic Distribution of Fibers and Glands in Nasal Polyps
- Electron-microscopic Findings of Elastic Fibers in Zebrafish Skin