J Korean Neurosurg Soc.
2014 May;55(5):244-247. 10.3340/jkns.2014.55.5.244.
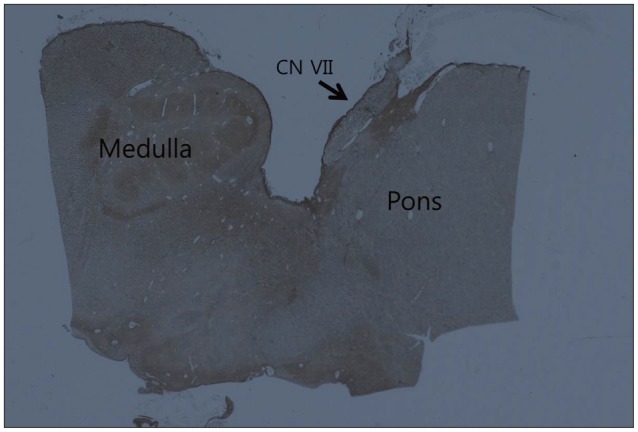
Microanatomy and Histological Features of Central Myelin in the Root Exit Zone of Facial Nerve
- Affiliations
-
- 1Department of Neurosurgery, Gil Medical Center, Gachon University, Incheon, Korea. gtyee@gilhospital.com
- 2Deparment of Neurosurgery, Ilsan Paik Hospital, College of Medicine, Inje University, Goyang, Korea.
- KMID: 2018028
- DOI: http://doi.org/10.3340/jkns.2014.55.5.244
Abstract
OBJECTIVE
The aim of this study was to evaluate the microanatomy and histological features of the central myelin in the root exit zone of facial nerve.
METHODS
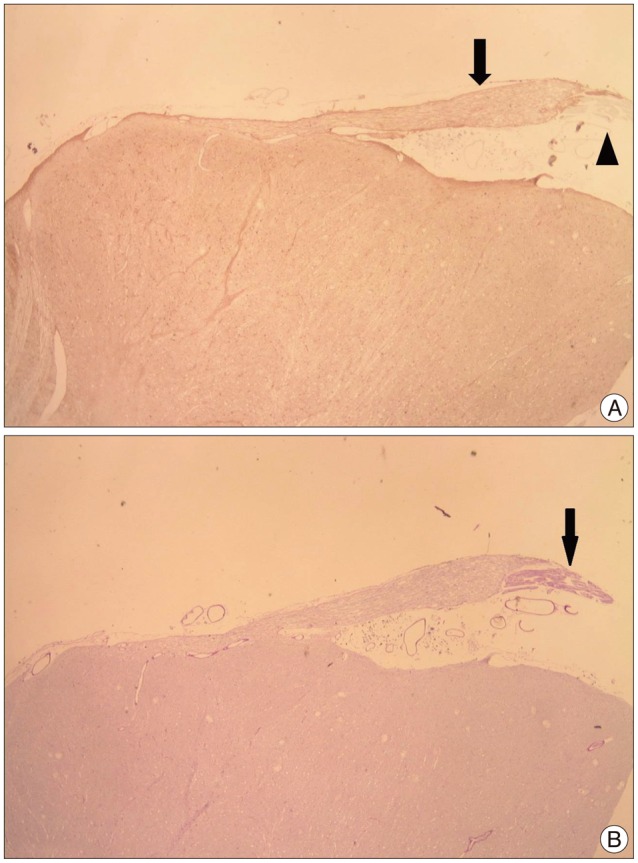
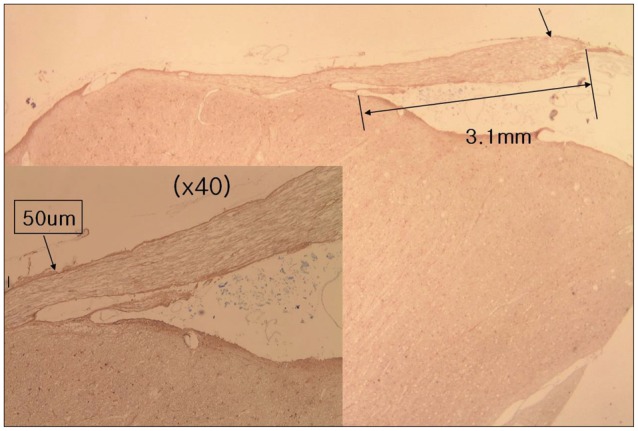
Forty facial nerves with brain stem were obtained from 20 formalin fixed cadavers. Among them 17 facial nerves were ruined during preparation and 23 root entry zone (REZ) of facial nerves could be examined. The length of medial REZ, from detach point of facial nerve at the brain stem to transitional area, and the thickness of glial membrane of central myelin was measured. We cut brain stem along the facial nerve and made a tissue block of facial nerve REZ. Each tissue block was embedded with paraffin and serially sectioned. Slices were stained with hematoxylin and eosin (H&E), periodic acid-Schiff, and glial fibrillary acid protein. Microscopy was used to measure the extent of central myelin and thickness of outer glial membrane of central myelin. Thickness of glial membrane was examined at two different points, the thickest area of proximal and distal REZ.
RESULTS
Special stain with PAS and GFAP could be differentiated the central and peripheral myelin of facial nerve. The length of medial REZ was mean 2.6 mm (1.6-3.5 mm). The glial limiting membrane of brain stem is continued to the end of central myelin. We called it glial sheath of REZ. The thickness of glial sheath was mean 66.5 microm (40-110 microm) at proximal REZ and 7.4 microm (5-10 microm) at distal REZ.
CONCLUSION
Medial REZ of facial nerve is mean 2.6 mm in length and covered by glial sheath continued from glial limiting membrane of brain stem. Glial sheath of central myelin tends to become thin toward transitional zone.
Keyword
MeSH Terms
Figure
Reference
-
1. Adams CB. Microvascular compression : an alternative view and hypothesis. J Neurosurg. 1989; 70:1–12. PMID: 2642544.2. De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L. Is the root entry/exit zone important in microvascular compression syndromes? Neurosurgery. 2002; 51:427–433. discussion 433-434. PMID: 12182781.
Article3. Gardner WJ. Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg. 1962; 19:947–958. PMID: 13946557.
Article4. Gardner WJ. Cross talk-the paradoxical transmission of a nerve impulse. Arch Neurol. 1966; 14:149–156. PMID: 4378157.
Article5. Henschen F. Zur Histologie und Pathogenese der Kleinhirnbrückenwinkeltumoren. Arch Psychiat Nervenkr. 1915; 56:21–122.
Article6. Jannetta PJ. The cause of hemifacial spasm : definitive microsurgical treatment at the brainstem in 31 patients. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1975; 80(3 Pt 1):319–322.7. Jannetta PJ, Abbasy M, Maroon JC, Ramos FM, Albin MS. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J Neurosurg. 1977; 47:321–328. PMID: 894338.8. McFarland DE, Friede RL. Number of fibres per sheath cell and internodal length in cat cranial nerves. J Anat. 1971; 109(Pt 1):169–176. PMID: 5556669.9. Møller AR, Jannetta PJ. On the origin of synkinesis in hemifacial spasm results of intracranial recordings. J Neurosurg. 1984; 61:569–576. PMID: 6086858.
Article10. Nakazawa E, Ishikawa H. Ultrastructural observations of astrocyte end-feet in the rat central nervous system. J Neurocytol. 1998; 27:431–440. PMID: 10192524.11. Peker S, Kurtkaya O, Uzün I, Pamir MN. Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery. 2006; 59:354–359. discussion 354-359. PMID: 16883175.
Article12. Skinner HA. Some histologic features of the cranial nerves. Arch Neurol Psychiatry. 1931; 25:356–372.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Hemifacial Spasm Caused by an Artery Passing Through the Facial Nerve
- Significance of Arachnoid Dissection to Obtain Optimal Exposure of Lower Cranial Nerves and the Facial Nerve Root Exit Zone during Microvascular Decompression Surgery
- Physical Facial Nerve Block in the Treatment of Hemifacial spasm
- Treatment of Venous Origin Hemifacial Spasm by Microsurgical Decompression: Case Report
- Research on the Pathogenesis of Hemifacial Spasm through Electrophysiological Studies