J Korean Fract Soc.
2012 Apr;25(2):142-145. 10.12671/jkfs.2012.25.2.142.
Delayed Foreign-body Reaction of Ankle Fracture Treated with a Biodegradable Plate and Screws: A Case Report
- Affiliations
-
- 1Department of Orthopaedic Surgery, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 2Department of Pathology, The Armed Forces Capital Hospital, Seongnam, Korea.
- 3Department of Orthopedic Surgery, The Armed Forces Capital Hospital, Seongnam, Korea. hohotoy@nate.com
- KMID: 2015458
- DOI: http://doi.org/10.12671/jkfs.2012.25.2.142
Abstract
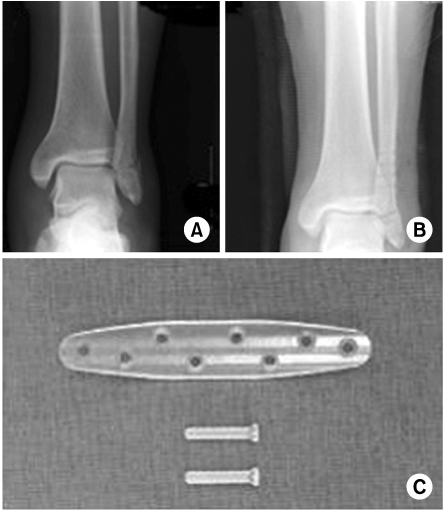
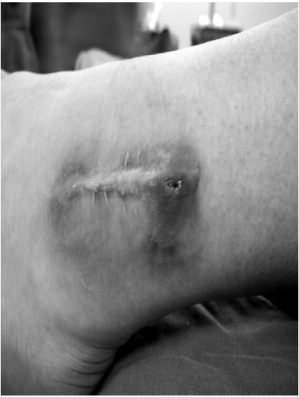
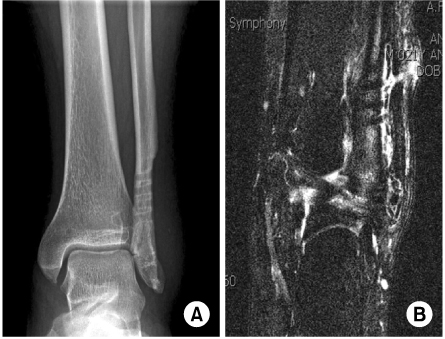
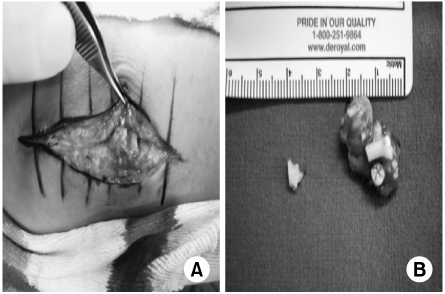
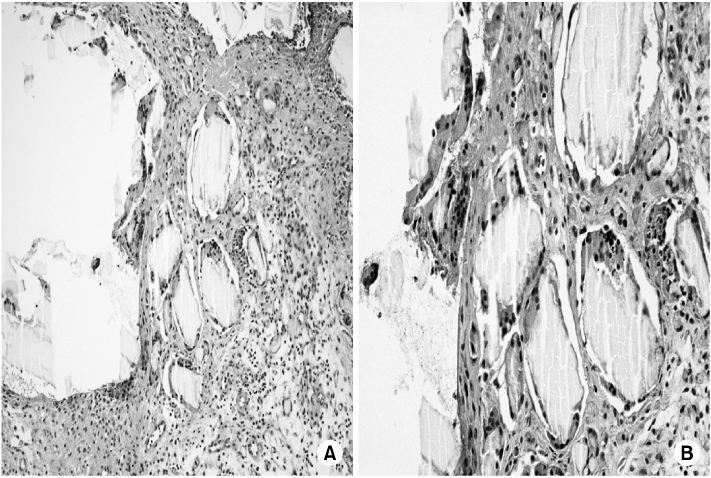
- Biodegradable implants made of co-polymers composed of L-lactide, D-lactide, and trimethylene carbonate were used in the present case. To our knowledge, only one reported tissue reaction has been associated with ankle fracture treated with third-generation implants internationally and none yet domestically. We report a delayed foreign-body reaction of ankle fracture treated with a third-generation biodegradable plate and screws. We suggest that ankle fracture patients treated with biodegradable implants should be advised of this possible complication and should be followed for at least 2 years.
Keyword
Figure
Reference
-
1. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000. 21:2615–2621.
Article2. Cho JY, Kim JW, Kim SE, Jung KC, Choi SH. Surgical fixation with biodegradable plate for the treatment of ankle fractures. J Korean Fract Soc. 2008. 21:31–36.
Article3. Kukk A, Nurmi JT. A retrospective follow-up of ankle fracture patients treated with a biodegradable plate and screws. Foot Ankle Surg. 2009. 15:192–197.
Article4. Laughlin RM, Block MS, Wilk R, Malloy RB, Kent JN. Resorbable plates for the fixation of mandibular fractures: a prospective study. J Oral Maxillofac Surg. 2007. 65:89–96.
Article5. Losken HW, van Aalst JA, Mooney MP, et al. Biodegradation of Inion fast-absorbing biodegradable plates and screws. J Craniofac Surg. 2008. 19:748–756.
Article6. Mavrogenis AF, Kanellopoulos AD, Nomikos GN, Papagelopoulos PJ, Soucacos PN. Early experience with biodegradable implants in pediatric patients. Clin Orthop Relat Res. 2009. 467:1591–1598.
Article7. Nieminen T, Rantala I, Hiidenheimo I, et al. Degradative and mechanical properties of a novel resorbable plating system during a 3-year follow-up in vivo and in vitro. J Mater Sci Mater Med. 2008. 19:1155–1163.
Article8. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000. 21:2607–2613.
Article9. Wood GD. Inion biodegradable plates: the first century. Br J Oral Maxillofac Surg. 2006. 44:38–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Clinical Study of Biodegradable Plates and Screws in Oral and Maxillofacial Surgery
- A Stainless Steel Foreign body in the Knee Joint With Synovitis: A Case Report
- The Result of Mandible Fracture Fixations with Biodegradable Materials
- Delayed Foreign Body Reaction Caused by Bioabsorbable Plates Used for Maxillofacial Fractures
- Pitfalls in Treatment of Lateral Malleolar Frcture with Plate and Screws