Anat Cell Biol.
2012 Jun;45(2):79-85. 10.5115/acb.2012.45.2.79.
Reappraisal of intergender differences in the urethral striated sphincter explains why a completely circular arrangement is difficult in females: a histological study using human fetuses
- Affiliations
-
- 1Department of Urology, National Hospital Organization Higashi-Hiroshima Medical Center, Higashi-Hiroshima, Japan. masumoti@hotmail.co.jp
- 2Department of Urology, Tottori University School of Medicine, Yonago, Japan.
- 3Department of Anatomy and Embryology II, Faculty of Medicine, Universidad Complutense, Madrid, Spain.
- 4Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- 5Department of Urology, Graduate School of Biomedical Sciences, Hiroshima University, Hiroshima, Japan.
- KMID: 2005869
- DOI: http://doi.org/10.5115/acb.2012.45.2.79
Abstract
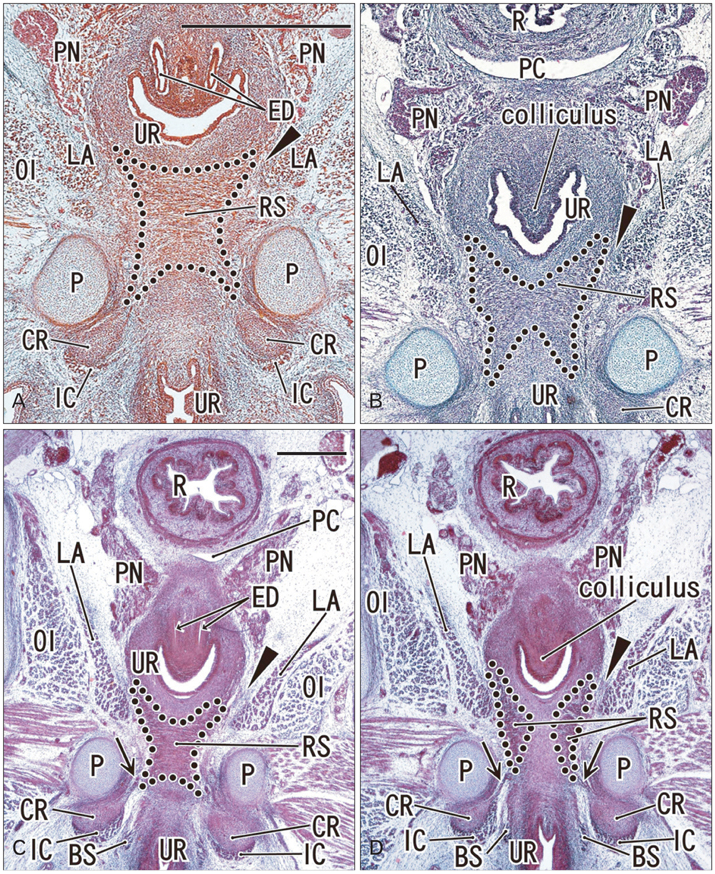
- To investigate why the development of a completely circular striated sphincter is so rare, we examined histological sections of 11 female and 11 male mid-term human fetuses. In male fetuses, the striated muscle initially extended in the frontal, rather than in the horizontal plane. However, a knee-like portion was absent in the female fetal urethra because, on the inferior side of the vaginal end, a wide groove for the future vestibule opened inferiorly. Accordingly, it was difficult for the developing striated muscle to surround the groove, even though there was not a great difference in width or thickness between the female vestibule and the male urethra. The development of a completely circular striated sphincter seems to be impossible in females because of interruption of the frontal plane by the groove-like vestibule. However, we cannot rule out the possibility that before descent of the vagina, the urethral striated muscle extends posteriorly.
Figure
Reference
-
1. Strasser H, Ninkovic M, Hess M, Bartsch G, Stenzl A. Anatomic and functional studies of the male and female urethral sphincter. World J Urol. 2000. 18:324–329.2. Kurihara M, Murakami G, Kajiwara M, Taguchi K, Tsukamoto T, Usui T. Lack of the complete circular rhabdosphincter and a distinct circular smooth muscle layer around the proximal urethra in elderly Japanese women: an anatomical study. Int Urogynecol J Pelvic Floor Dysfunct. 2004. 15:85–94.3. Perucchini D, DeLancey JO, Ashton-Miller JA, Galecki A, Schaer GN. Age effects on urethral striated muscle. II. Anatomic location of muscle loss. Am J Obstet Gynecol. 2002. 186:356–360.4. Sebe P, Fritsch H, Oswald J, Schwentner C, Lunacek A, Bartsch G, Radmayr C. Fetal development of the female external urinary sphincter complex: an anatomical and histological study. J Urol. 2005. 173:1738–1742.5. Masumoto H, Rodríguez-Vázquez JF, Verdugo-López S, Murakami G, Matsubara A. Fetal topographical anatomy of the female urethra and descending vagina: a histological study of the early human fetal urethra. Ann Anat. 2011. 193:500–508.6. Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983. 205:223–232.7. Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, Usui T. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008. 19:1663–1670.8. Kato M, Niikura H, Yaegashi N, Murakami G, Tatsumi H, Matsubara A. Histotopography of the female cavernous nerve: a study using donated fetuses and adult cadavers. Int Urogynecol J Pelvic Floor Dysfunct. 2008. 19:1687–1695.9. O'Rahilly R, Müller F. Human embryology and teratology. 1996. 2nd ed. New York: Wiley-Liss.10. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, Kudo Y. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011. 24:469–477.11. Hirata E, Koyama M, Murakami G, Ohtsuka A, Abe S, Ide Y, Fujiwara H, Kudo Y. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011. 37:13–23.12. Valasek P, Evans DJ, Maina F, Grim M, Patel K. A dual fate of the hindlimb muscle mass: cloacal/perineal musculature develops from leg muscle cells. Development. 2005. 132:447–458.13. Sasaki C, Yamaguchi K, Akita K. Spatiotemporal distribution of apoptosis during normal cloacal development in mice. Anat Rec A Discov Mol Cell Evol Biol. 2004. 279:761–767.14. Yamaguchi K, Kiyokawa J, Akita K. Developmental processes and ectodermal contribution to the anal canal in mice. Ann Anat. 2008. 190:119–128.15. Arakawa T, Murakami G, Nakajima F, Matsubara A, Ohtsuka A, Goto T, Teramoto T. Morphologies of the interfaces between the levator ani muscle and pelvic viscera, with special reference to muscle insertion into the anorectum in elderly Japanese. Anat Sci Int. 2004. 79:72–81.16. Nakajima F, Takenaka A, Uchiyama E, Hata F, Suzuki D, Murakami G. Macroscopic and histotopographic study of the deep transverse perineal muscle (musculus transversus perinei profundus) in elderly Japanese. Ann Anat. 2007. 189:65–74.17. Oelrich TM. The urethral sphincter muscle in the male. Am J Anat. 1980. 158:229–246.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anatomical Features of Male Rat Urethra and Comparison of Urethral Sphincter Contractility according to Different Urethral Strip Orientations
- Clinical and Functional Anatomy of the Urethral Sphincter
- Effect of Transrectal Probe Insertion on the Opening of Internal Urethral Sphincter
- Dynamic Urethral Pressure Profilometry using Triple Lumen Urodynamic Catheter
- Ultrastructural Study on Transitional Zone of Esophageal Skeleto-Smooth Muscle in Rodents