J Korean Soc Radiol.
2015 Feb;72(2):108-114. 10.3348/jksr.2015.72.2.108.
The Effects of Proteoglycan and Type II Collagen on T1rho Relaxation Time of Articular Cartilage
- Affiliations
-
- 1Department of Radiology and Institute of Radiation Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. dalnara3@gmail.com
- KMID: 2002806
- DOI: http://doi.org/10.3348/jksr.2015.72.2.108
Abstract
- PURPOSE
To evaluate the effects of proteoglycan and type II collagen within articular cartilage on T1rho relaxation time of articular cartilage.
MATERIALS AND METHODS
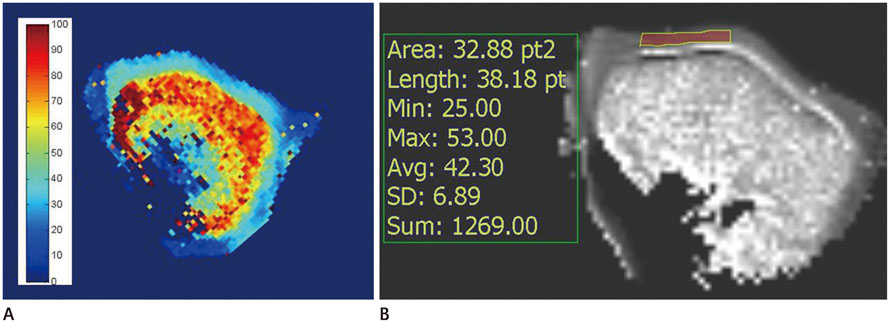
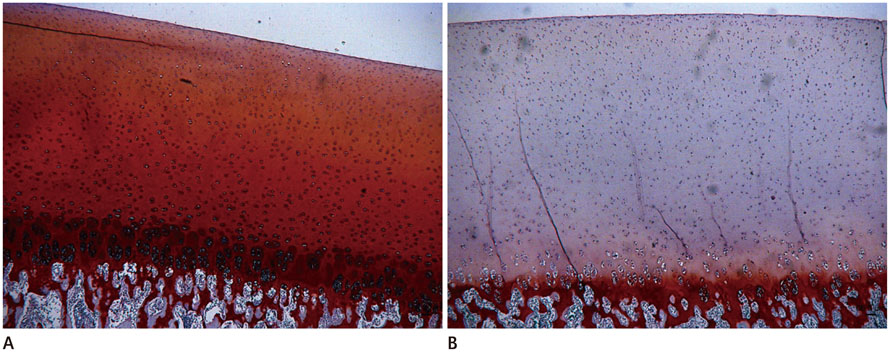
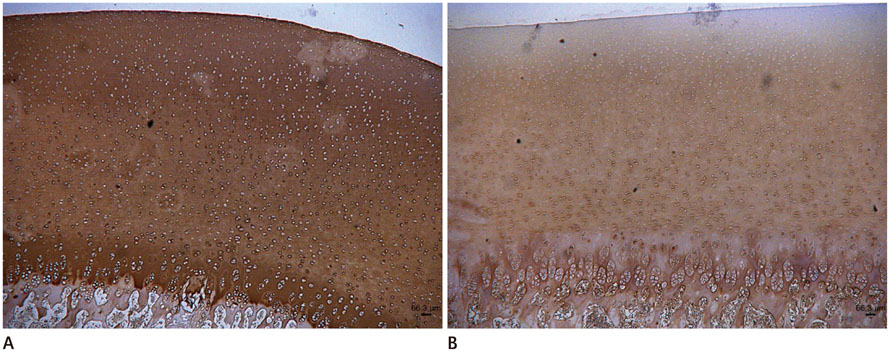
This study was exempted by the institutional and animal review boards, and informed consent was not required. Twelve porcine patellae were assigned to three groups of control, trypsin-treated (proteoglycan-degraded), or collagenase-treated (collagen-degraded). The T1rho images were obtained with a 3 tesla magnetic resonance imaging scanner with a single loop coil. Statistical differences were detected by analysis of variance to evaluate the effects of the enzyme on T1rho relaxation time. Safranin-O was used to stain proteoglycan in the articular cartilage and immunohistochemical staining was performed for type II collagen.
RESULTS
Mean T1rho values of the control, trypsin-treated, and collagenase-treated groups were 37.72 +/- 5.82, 57.53 +/- 8.24, and 45.08 +/- 5.31 msec, respectively (p < 0.001). Histology confirmed a loss of proteoglycan and type II collagen in the trypsin- and collagenase-treated groups.
CONCLUSION
Degradation of proteoglycans and collagen fibers in the articular cartilage increased the articular cartilage T1rho value.
MeSH Terms
Figure
Reference
-
1. Shapiro LM, McWalter EJ, Son MS, Levenston M, Hargreaves BA, Gold GE. Mechanisms of osteoarthritis in the knee: MR imaging appearance. J Magn Reson Imaging. 2014; 39:1346–1356.2. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998; 47:487–504.3. Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998; 41:1331–1342.4. Burstein D, Gray M, Mosher T, Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am. 2009; 47:675–686.5. Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997; 205:551–558.6. Taylor C, Carballido-Gamio J, Majumdar S, Li X. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magn Reson Imaging. 2009; 27:779–784.7. Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007; 15:789–797.8. Nishioka H, Hirose J, Nakamura E, Oniki Y, Takada K, Yamashita Y, et al. T1ρ and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012; 35:147–155.9. Stahl R, Luke A, Li X, Carballido-Gamio J, Ma CB, Majumdar S, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients--a 3.0-Tesla MRI study. Eur Radiol. 2009; 19:132–143.10. Trattnig S, Mlynárik V, Breitenseher M, Huber M, Zembsch A, Rand T, et al. MRI visualization of proteoglycan depletion in articular cartilage via intravenous administration of Gd-DTPA. Magn Reson Imaging. 1999; 17:577–583.11. Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000; 214:259–266.12. Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004; 232:592–598.13. Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T1ρ and T2 relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage. 2013; 21:69–76.14. Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001; 46:419–423.15. Mlynárik V, Trattnig S, Huber M, Zembsch A, Imhof H. The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. J Magn Reson Imaging. 1999; 10:497–502.16. Borthakur A, Shapiro EM, Beers J, Kudchodkar S, Kneeland JB, Reddy R. Sensitivity of MRI to proteoglycan depletion in cartilage: comparison of sodium and proton MRI. Osteoarthritis Cartilage. 2000; 8:288–293.17. Shibata S, Fukada K, Suzuki S, Yamashita Y. Immunohistochemistry of collagen types II and X, and enzyme-histochemistry of alkaline phosphatase in the developing condylar cartilage of the fetal mouse mandible. J Anat. 1997; 191(Pt 4):561–570.18. Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997; 38:863–867.19. Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011; 29:324–334.20. Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004; 51:503–509.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene Delivery into Chondrocytes by Liposome
- Immunohistochemical Staining for Type II Collagen in Regenerated Cartilage after Microfracture Surgery
- The Effects of Air Exposure on Articular Cartilage ; Degeneration & Regeneration
- Effects of human serum and TGF-beta on proliferation and redifferentiation of human articular chondrocytes
- Effects of IGF (Insulin-like growth factor) on sub-populated articular chondrocytes by Percoll density gradient