Korean J Urol.
2007 Nov;48(11):1125-1130. 10.4111/kju.2007.48.11.1125.
The Effect of Neoadjuvant Hormonal Treatment in Prostate Cancer on Biochemical Recurrence
- Affiliations
-
- 1Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. cskim@amc.seoul.kr
- KMID: 1997088
- DOI: http://doi.org/10.4111/kju.2007.48.11.1125
Abstract
-
PURPOSE: When combined with surgery, neoadjuvant hormonal therapy (NHT) has not demonstrated a significant benefit for meaningful clinical endpoints such as progression-free survival or overall survival. We evaluated the effect of NHT on prostate cancer.
MATERIALS AND METHODS
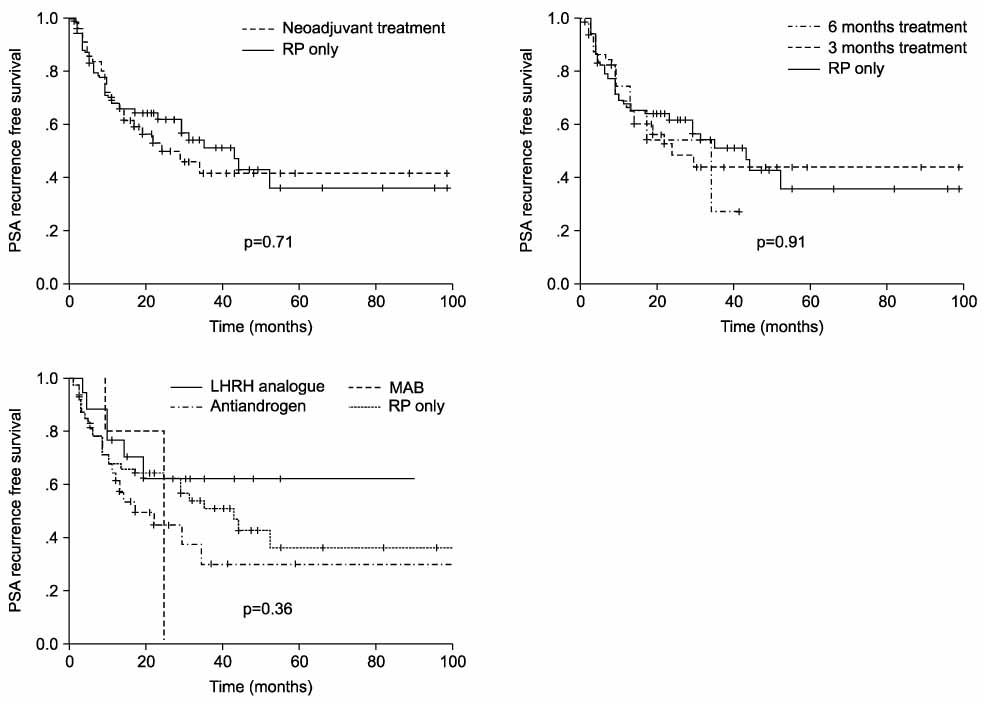
From 1995 to 2004, 519 patients underwent radical retropubic prostatectomy(RRP). One-hundred thirty of them were included in this retrospective case-control study and they were assessed for age, the preoperative prostate-specific antigen(PSA) level, the clinical stage and the biopsy Gleason score(GS). The subjects were divided into two groups: the RRP only group(n=65) and the NHT group(n=65), and these were matched for the 3 above mentioned parameters. The protocols for NHT were maximal androgen blockade(n=40), antiandrogen only(n=8), and LHRH analogue only(n=17). Biochemical recurrence was defined as a level of serum PSA of 0.2ng/ml or greater on 2 consecutive evaluations.
RESULTS
The mean age of the RRP only group and the NHT group was 64.2 and 63.5, respectively(p>0.05). The rates of a positive surgical margin and biochemical recurrence in the NHT group were 49.2% and 42.5%, respectively, and they were 46.2% and 46.2%, respectively, in RRP only group, and there was no statistical difference between the two groups. In high risk patients(clinical stage> or =T3, biopsy GS> or =8, serum PSA>20ng/ml), NHT group was not differences compared with the RRP group. Neither the duration (3 months vs. 6 months) of NHT nor the regimens of NHT improved the clinical and surgical outcome.
CONCLUSIONS
NHT did not improve biochemical recurrence and the positive surgical margin.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006. 56:106–130.2. Cancer Registration and Biostatistics Branch, National Cancer Center. Cancer Statistics in Korea. 2003.3. Rosen MA, Goldstone L, Lapin S, Wheeler T, Scardino PT. Frequency and location of extracapsular extension and positive surgical margins in radical prostatectomy specimens. J Urol. 1992. 148:331–337.4. Bigg SW, Kavoussi LR, Catalona WJ. Role of nerve-sparing radical prostatectomy for clinical stage B2 prostate cancer. J Urol. 1990. 144:1420–1424.5. Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001. 166:500–506.6. Song J, Chang K. Current status of hormonal therapy for prostate cancer in Korea: a national survey of Korean urooncologists. Korean J Urol Oncol. 2003. 1:175–180.7. Park EK, Chung BH, Hong SJ. Influences of neoadjuvant androgen ablation before radical prostatectomy on positive surgical margin and biochemical recurrence rate. Korean J Urol. 2004. 45:518–523.8. Jones EC. Resection margin status in radical retropubic prostatectomy specimens: relationship to type of operation, tumor size, tumor grade and local tumor extension. J Urol. 1990. 144:89–93.9. Rosen MA, Goldstone L, Lapin S, Wheeler T, Scardino PT. Frequency and location of extracapsular extension and positive surgical margins in radical prostatectomy specimens. J Urol. 1992. 148:331–337.10. Partin AW, Yoo J, Carter HB, Pearson JD, Chan DW, Epstein JI, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993. 150:110–114.11. Lee HH, Warde P, Jewett MA. Neoadjuvant hormonal therapy in carcinoma of the prostate. BJU Int. 1999. 83:438–448.12. Pilepich MV, Krall JM, al-Sarraf M, John MJ, Doggett RL, Sause WT, et al. Androgen deprivation with radiation therapy compared with radiation therapy alone for locally advanced prostatic carcinoma: a randomized comparative trial of the Radiation Therapy Oncology Group. Urology. 1995. 45:616–623.13. Schulman CC. Neoadjuvant androgen blockade prior to prostatectomy: a retrospective study and critical review. Prostate Suppl. 1994. 5:9–14.14. Soloway MS, Sharifi R, Wajsman Z, McLeod D, Wood DP Jr, Puras-Baez A. The Lupron Depot Neoadjuvant Prostate Cancer Study Group. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. J Urol. 1995. 154:424–428.15. Goldenberg SL, Klotz LH, Srigley J, Jewett MA, Mador D, Fradet Y, et al. Canadian Urologic Oncology Group. Randomized, prospective, controlled study comparing radical prostatectomy alone and neoadjuvant androgen withdrawal in the treatment of localized prostate cancer. J Urol. 1996. 156:873–877.16. Swindle P, Eastham JA, Ohori M, Kattan MW, Wheeler T, Maru N, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005. 174:903–907.17. Klotz LH, Goldenberg SL, Jewett M, Barkin J, Chetner M, Fradet Y, et al. Canadian Urologic Oncology Group. CUOG randomized trial of neoadjuvant androgen ablation before radical prostatectomy: 36-month post-treatment PSA results. Urology. 1999. 53:757–763.18. Meyer F, Moore L, Bairati I, Lacombe L, Tetu B, Fradet Y. Neoadjuvant hormonal therapy before radical prostatectomy and risk of prostate specific antigen failure. J Urol. 1999. 162:2024–2028.19. Lorente JA, Arango O, Bielsa O, Cortadellas R, Lloreta-Trull J, Gelabert-Mas A. A longer duration of neo-adjuvant combined androgen blockade prior to radical prostatectomy may lead to lower tumour volume of localised prostate cancer. Eur Urol. 2003. 43:119–123.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influences of Neoadjuvant Androgen Ablation before Radical Prostatectomy on Positive Surgical Margin and Biochemical Recurrence Rate
- Neoadjuvant Hormonal Therapy Preceding Radical Prostatectomy for Clinically Localized Prostate Cancer: Early Postoperative Complications and Biochemical Recurrence
- Quality of Life in Prostate Cancer Patient Undergoing Androgen Deprivation Therapy
- Outcome of Radical Prostatectomy in Prostate Cancer Patients with Prostate-Specific Antigen (PSA) Level Equal to or More Than 20 ng/ml and No Distant Metastasis Preoperatively
- Significance of Neoadjuvant Hormonal Therapy in Radical Retropubic Prostatectomy: A Retrospective Single-Surgeon Study