J Korean Soc Radiol.
2015 Jan;72(1):1-10. 10.3348/jksr.2015.72.1.1.
Differentiation of Common Pancreatic Cystic Neoplasms Based Upon Multiplicity of Cysts
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jhbyun@amc.seoul.kr
- 2Department of Radiology, Eulji Hospital, Eulji University School of Medicine, Seoul, Korea.
- KMID: 1823928
- DOI: http://doi.org/10.3348/jksr.2015.72.1.1
Abstract
- PURPOSE
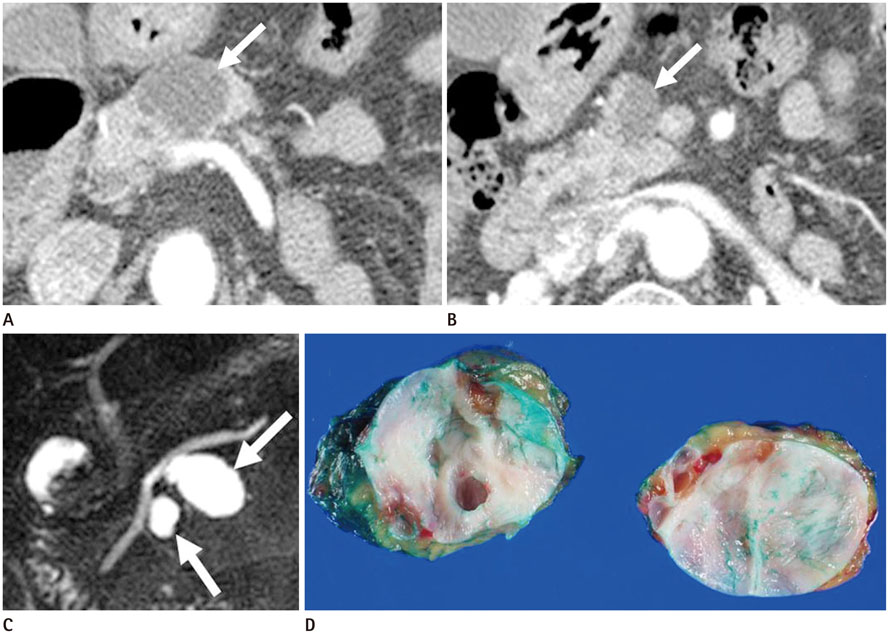
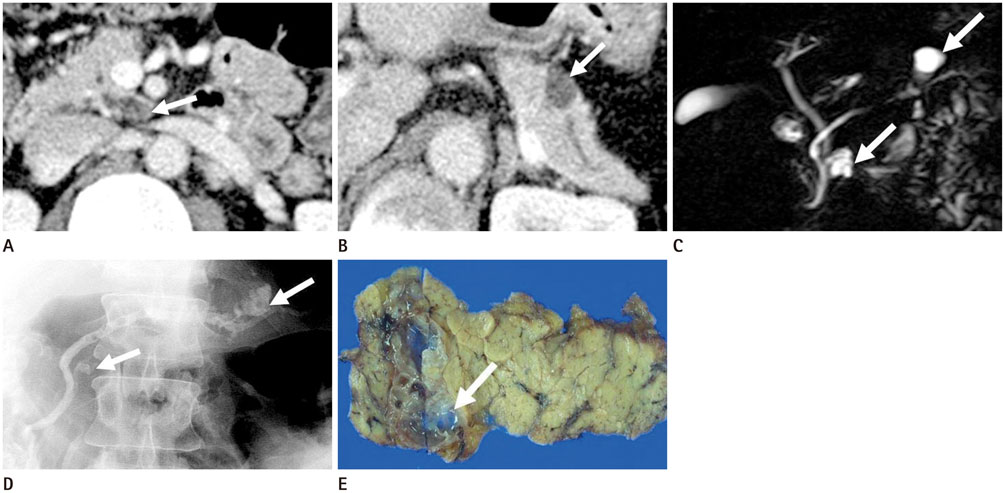
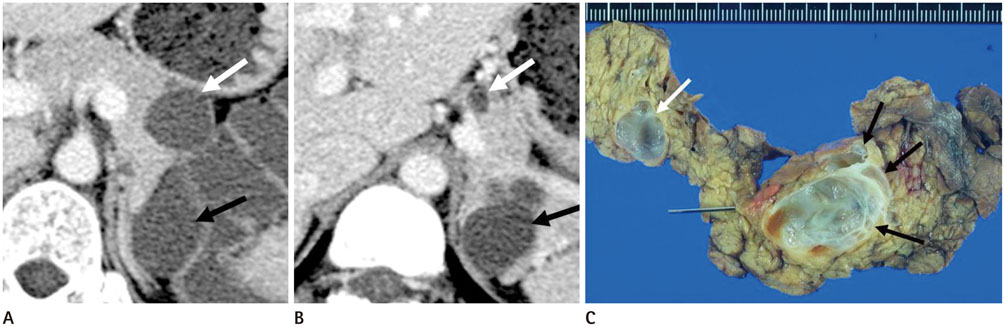
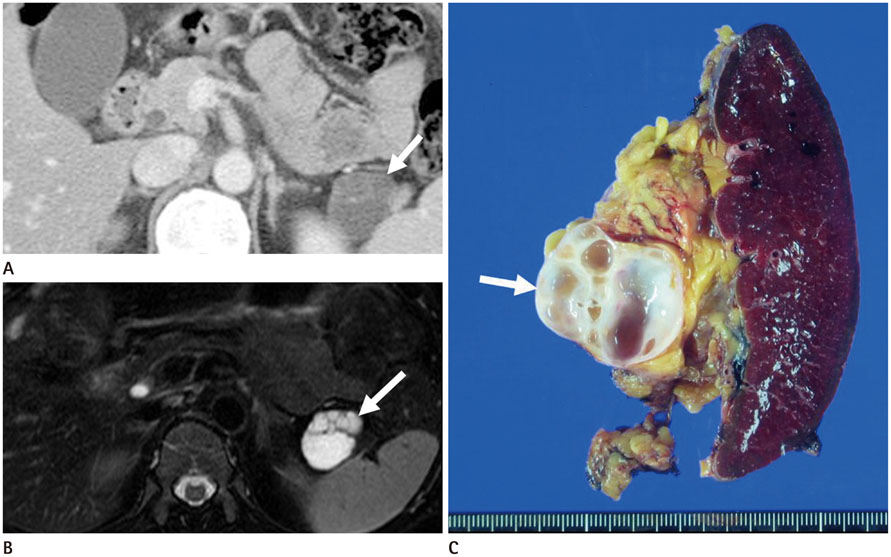
To evaluate the multiplicity and mean number of cystic lesions in patients with branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), serous cystadenomas (SCAs), and mucinous cystic neoplasms (MCNs) of the pancreas.
MATERIALS AND METHODS
Two hundred and eighty-eight patients with pathologically proven cystic neoplasms of the pancreas underwent preoperative CT and/or MRI. These patients were divided into the following three groups: BD-IPMN, SCA, and MCN groups. Two radiologists retrospectively analyzed the CT and MRI examinations to determine the multiplicity (i.e., more than one lesion) and mean number of cystic lesions per patient. Among the three groups, statistical comparison of multiplicity of cystic lesions was performed.
RESULTS
The BD-IPMN group consisted of 155 patients with 176 BD-IPMNs, with multiplicity in 15 patients (9.7%) and a mean 1.14 BD-IPMNs per patient (range, 1.3). The SCA group consisted of 67 patients with 69 SCAs, with multiplicity in one patient (1.5%) and a mean 1.03 SCAs per patient (range, 1.3). The MCN group consisted of 67 patients with 67 MCNs, with no multiplicity. Multiple cystic lesions were significantly more common in the BD-IPMN group than in the SCA and MCN groups (p = 0.003).
CONCLUSION
BD-IPMNs were more frequently associated with multiplicity of cystic lesions than SCAs or MCNs of the pancreas.
MeSH Terms
Figure
Reference
-
1. Fernández-del Castillo C, Warshaw AL. Cystic tumors of the pancreas. Surg Clin North Am. 1995; 75:1001–1016.2. Federle MP, McGrath KM. Cystic neoplasms of the pancreas. Gastroenterol Clin North Am. 2007; 36:365–376. ix3. Campbell F, Azadeh B. Cystic neoplasms of the exocrine pancreas. Histopathology. 2008; 52:539–551.4. Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas. 2004; 28:282–288.5. Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002; 123:1500–1507.6. Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004; 239:678–685. discussion 685-687.7. Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003; 90:1244–1249.8. Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006; 6:17–32.9. Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007; 56:1086–1090.10. Irie H, Yoshimitsu K, Aibe H, Tajima T, Nishie A, Nakayama T, et al. Natural history of pancreatic intraductal papillary mucinous tumor of branch duct type: follow-up study by magnetic resonance cholangiopancreatography. J Comput Assist Tomogr. 2004; 28:117–122.11. Hutchins GF, Draganov PV. Cystic neoplasms of the pancreas: a diagnostic challenge. World J Gastroenterol. 2009; 15:48–54.12. Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005; 25:1471–1484.13. Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000; 175:99–103.14. Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999; 23:906–912.15. Song SJ, Lee JM, Kim YJ, Kim SH, Lee JY, Han JK, et al. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: comparison of multirow-detector CT and MR imaging using ROC analysis. J Magn Reson Imaging. 2007; 26:86–93.16. Choi EK, Park SH, Kim DY, Kim KW, Byun JH, Lee MG, et al. Unusual manifestations of primary pancreatic neoplasia: Radiologic-pathologic correlation. J Comput Assist Tomogr. 2006; 30:610–617.17. Procacci C, Graziani R, Bicego E, Bergamo-Andreis IA, Guarise A, Valdo M, et al. Serous cystadenoma of the pancreas: report of 30 cases with emphasis on the imaging findings. J Comput Assist Tomogr. 1997; 21:373–382.18. Procacci C, Graziani R, Bicego E, Bergamo-Andreis IA, Mainardi P, Zamboni G, et al. Intraductal mucin-producing tumors of the pancreas: imaging findings. Radiology. 1996; 198:249–257.19. Johnson CD, Stephens DH, Charboneau JW, Carpenter HA, Welch TJ. Cystic pancreatic tumors: CT and sonographic assessment. AJR Am J Roentgenol. 1988; 151:1133–1138.20. Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, et al. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006; 187:1192–1198.21. Cohen-Scali F, Vilgrain V, Brancatelli G, Hammel P, Vullierme MP, Sauvanet A, et al. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology. 2003; 228:727–733.22. Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas: diagnosis and treatment. Surgery. 1997; 122:617–625.23. Procacci C, Megibow AJ, Carbognin G, Guarise A, Spoto E, Biasiutti C, et al. Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics. 1999; 19:1447–1463.24. Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999; 230:152–161.25. Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, Kimura W, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004; 28:241–246.26. Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol. 2007; 20:Suppl 1. S71–S93.27. Lim JH, Yoon KH, Kim SH, Kim HY, Lim HK, Song SY, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004; 24:53–66. discussion 66-67.28. Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007; 102:1759–1764.