Yonsei Med J.
2010 Jul;51(4):546-551. 10.3349/ymj.2010.51.4.546.
Consolidations in Nodular Bronchiectatic Mycobacterium Avium Complex Lung Disease: Mycobacterium Avium Complex or Other Infection?
- Affiliations
-
- 1Department of Radiology, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. sghnk@catholic.ac.kr
- KMID: 1805192
- DOI: http://doi.org/10.3349/ymj.2010.51.4.546
Abstract
- PURPOSE
The objective of this study is to define the clinical implications of consolidations in nodular bronchiectatic type Mycobacterium avium complex (NB-MAC) infection.
MATERIALS AND METHODS
A total of 69 patients (M : F = 17 : 52; mean age, 64 years; age range, 41-85 years) with MAC isolated in the sputum culture and nodular bronchiectasis on the initial and follow-up CT scans were included. We retrospectively reviewed the incidence of consolidation and analyzed its clinical course by using radiographic changes with or without anti-MAC drug therapy.
RESULTS
In 44 of the 69 cases (64%), focal consolidations were seen on the initial and follow-up CT images. In 35 of the 44 (80%) cases, consolidations completely regressed, and in 3 cases (7%), consolidations partially regressed within 2 months with only antibiotics. In 2 cases (5%), the consolidations remained stable for over 2 months without anti-MAC drug therapy. Only in 4 cases (9%) did the consolidations improve after anti-MAC drug therapy. In 11 of the 38 cases (29%) with responsiveness to antibiotics, non-mycobacterial micro-organisms were identified in sputum, including pseudomonas, hemophilus, staphylococcus, and others.
CONCLUSION
In NB-MAC, consolidations are commonly present on CT. In these conditions, most of consolidations result from pneumonia other than MAC.
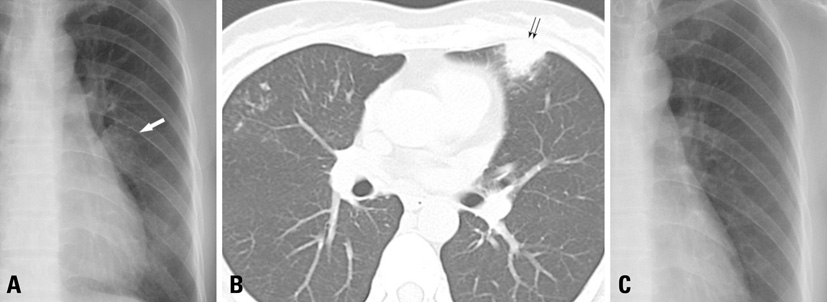
Figure
Reference
-
1. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997. 156:S1–S25.2. Koh WJ, Kwon OJ, Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol. 2002. 3:145–157.3. O'Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987. 135:1007–1014.4. Tsukamura M, Kita N, Shimoide H, Arakawa H, Kuze A. Studies on the epidemiology of nontuberculous mycobacteriosis in Japan. Am Rev Respir Dis. 1988. 137:1280–1284.5. Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989. 321:863–868.6. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992. 101:1605–1609.7. Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005. 235:282–288.
Article8. Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004. 231:880–886.
Article9. Kim TS, Koh WJ, Han J, Chung MJ, Lee JH, Lee KS, et al. Hypothesis on the evolution of cavitary lesions in nontuberculous mycobacterial pulmonary infection: thin-section CT and histopathologic correlation. AJR Am J Roentgenol. 2005. 184:1247–1252.10. Fujita J, Ohtsuki Y, Shigeto E, Suemitsu I, Yamadori I, Bandoh S, et al. Pathological findings of bronchiectases caused by Mycobacterium avium intracellulare complex. Respir Med. 2003. 97:933–938.11. Fujita J, Ohtsuki Y, Suemitsu I, Shigeto E, Yamadori I, Obayashi Y, et al. Pathological and radiological changes in resected lung specimens in Mycobacterium avium intracellulare complex disease. Eur Respir J. 1999. 13:535–540.12. Austin JH, Müller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996. 200:327–331.
Article13. Wickremasinghe M, Ozerovitch LJ, Davies G, Wodehouse T, Chadwick MV, Abdallah S, et al. Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax. 2005. 60:1045–1051.14. Okumura M, Iwai K, Ogata H, Ueyama M, Kubota M, Aoki M, et al. Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Intern Med. 2008. 47:1465–1472.15. Fujiuchi S, Matsumoto H, Yamazaki Y, Nakao S, Takahashi M, Satoh K, et al. Analysis of chest CT in patients with Mycobacterium avium complex pulmonary disease. Respiration. 2003. 70:76–81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid identification of mycobacterium avium and mycobacterium intracellulare by the amplification of rRNA sequences
- Respiratory Review of 2009: Nontuberculous Mycobacterium
- A Case of Pulmonary and Endobronchial Mycobacterium avium Infection Presenting as an Acute Pneumonia in an Immunocompetent Patient
- Vertebral Osteomyelitis due to Mycobacterium intracellulare in an Immunocompetent Elderly Patient After Vertebroplasty
- Disseminated Mycobacterium avium Complex Infection in a Patient with Acquired Immunodeficiency Syndrome