J Korean Med Sci.
2014 Jan;29(1):145-148. 10.3346/jkms.2014.29.1.145.
Localized Thymic Amyloidosis Presenting with Myasthenia Gravis: Case Report
- Affiliations
-
- 1Department of Pathology, Chungbuk National University College of Medicine, Cheongju, Korea. ok5218@hanmail.net
- 2Department of Thoracic and Cardiovascular Surgery, Chungbuk National University College of Medicine, Cheongju, Korea.
- KMID: 1796929
- DOI: http://doi.org/10.3346/jkms.2014.29.1.145
Abstract
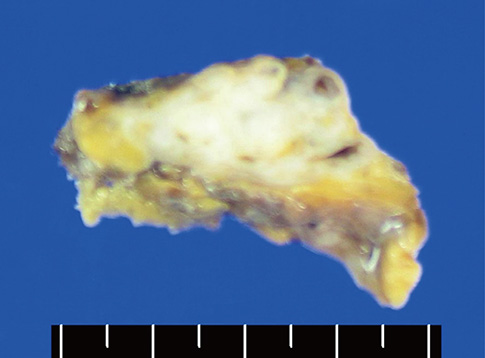
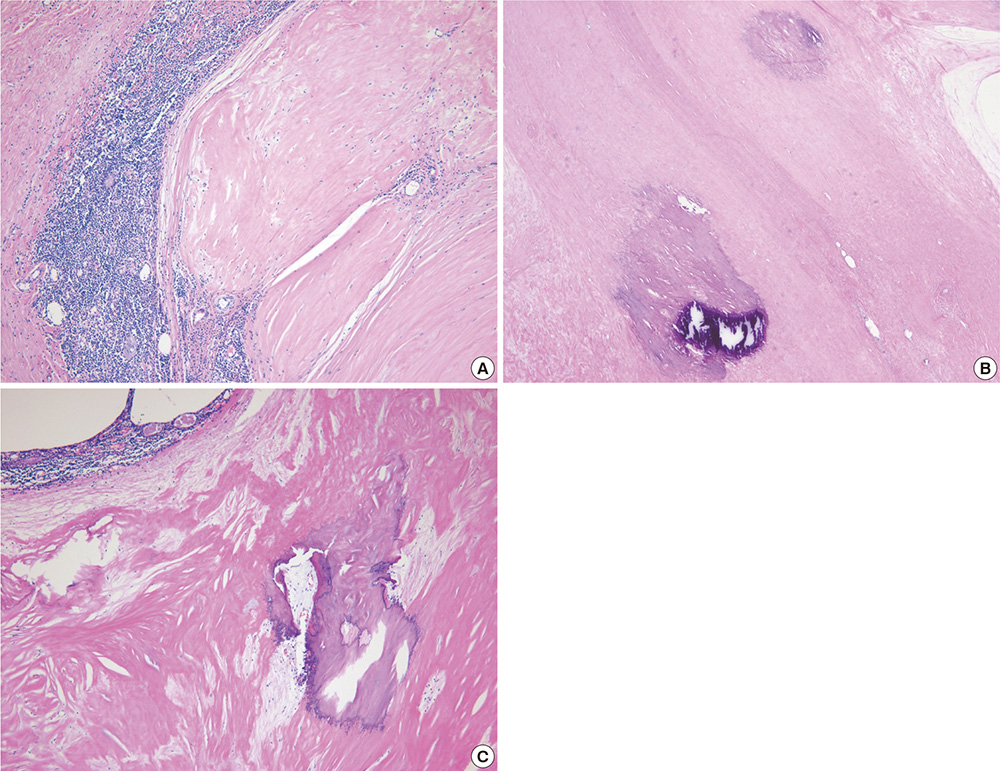
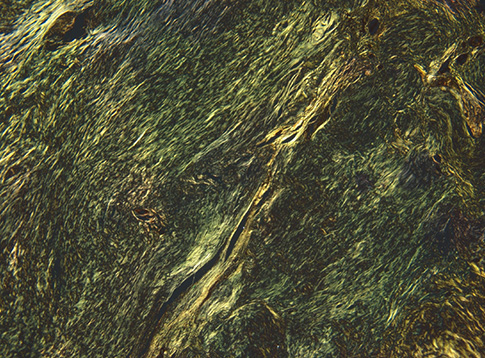
- A mediastinal mass was incidentally found on chest radiography in a 46-yr-old woman who had had myasthenia gravis (MG) for 2 months. Computed tomography revealed a 4-cm in size, well-defined, and lobulating mass with nodular calcification that was located in the thymus. Microscopically, the mass consisted of diffuse amorphous eosinophilic materials. These deposits exhibited apple-green birefringence under polarized light microscopy after Congo red staining. Immunohistochemical analysis revealed that they were positive for both kappa and lambda light chains and negative for amyloid A. A diagnosis of localized primary thymic amyloidosis was finally made. After thymectomy, the symptoms of MG were controlled with reduced corticosteroid requirements. Localized thymic amyloidosis associated with MG has not been reported to date.
Keyword
MeSH Terms
-
Amyloidosis/complications/*radiography/*surgery
Calcinosis/*radiography/*surgery
Female
Humans
Immunoglobulin kappa-Chains/immunology
Immunoglobulin lambda-Chains/immunology
Mediastinum/radiography/surgery
Middle Aged
Myasthenia Gravis/*complications
Radiography, Thoracic
Thymectomy
Thymus Gland/radiography/surgery
Tomography, X-Ray Computed
Immunoglobulin kappa-Chains
Immunoglobulin lambda-Chains
Figure
Reference
-
1. Nomenclature of amyloid and amyloidosis: WHO-IUIS Nomenclature Sub-Committee. Bull World Health Organ. 1993; 71:105–112.2. Ha SY, Lee JJ, Park HJ, Han JH, Kim HK, Lee KS. Localized primary thymic amyloidosis presenting as a mediastinal mass: a case report. Korean J Pathol. 2011; 45:Suppl 1. S41–S44.3. Kaplan B, Ramirez-Alvarado M, Sikkink L, Golderman S, Dispenzieri A, Livneh A, Gallo G. Free light chains in plasma of patients with light chain amyloidosis and non-amyloid light chain deposition disease: high proportion and heterogeneity of disulfide-linked monoclonal free light chains as pathogenic features of amyloid disease. Br J Haematol. 2009; 144:705–715.4. Bhat A, Selmi C, Naguwa SM, Cheema GS, Gershwin ME. Currents concepts on the immunopathology of amyloidosis. Clin Rev Allergy Immunol. 2010; 38:97–106.5. Wilson PG, Thompson JC, Webb NR, de Beer FC, King VL, Tannock LR. Serum amyloid A, but not C-reactive protein, stimulates vascular proteoglycan synthesis in a pro-atherogenic manner. Am J Pathol. 2008; 173:1902–1910.6. Wetmore JB, Lovett DH, Hung AM, Cook-Wiens G, Mahnken JD, Sen S, Johansen KL. Associations of interleukin-6, C-reactive protein and serum amyloid A with mortality in haemodialysis patients. Nephrology (Carlton). 2008; 13:593–600.7. Westermark GT, Westermark P. Serum amyloid A and protein AA: molecular mechanisms of a transmissible amyloidosis. FEBS Lett. 2009; 583:2685–2690.8. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003; 349:583–596.9. Urban BA, Fishman EK, Goldman SM, Scott WW Jr, Jones B, Humphrey RL, Hruban RH. CT evaluation of amyloidosis: spectrum of disease. Radiographics. 1993; 13:1295–1308.10. Pepys MB. Amyloidosis. Annu Rev Med. 2006; 57:223–241.11. Takamori S, Yano H, Hayashi A, Fukunaga M, Miwa K, Maeshiro K, Shirouzu K. Amyloid tumor in the anterior mediastinum: report of a case. Surg Today. 2004; 34:518–520.12. Siddachari RC, Chankar DA, Pramesh CS, Naresh KN, de Sousa CE, Dcruz AK. Laryngeal amyloidosis. J Otolaryngol. 2005; 34:60–63.13. Shah PL, Gillmore JD, Copley SJ, Collins JV, Wells AU, du Bois RM, Hawkins PN, Nicholson AG. The importance of complete screening for amyloid fibril type and systemic disease in patients with amyloidosis in the respiratory tract. Sarcoidosis Vasc Diffuse Lung Dis. 2002; 19:134–142.14. Anthony DC, Frosch MP, De Girolami U. Peripheral nerves and skeletal muscle. In : Schmitt W, editor. Robbins and Cotran pathologic basis of disease, professional edition. 8th ed. Philadelphia: Saunders;2008. p. 1275–1276.15. Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006; 116:2843–2854.16. Hohlfeld R, Toyka KV, Heininger K, Grosse-Wilde H, Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984; 310:244–246.17. Morgutti M, Conti-Tronconi BM, Sghirlanzoni A, Clementi F. Cellular immune response to acetylcholine receptor in myasthenia gravis: II. thymectomy and corticosteroids. Neurology. 1979; 29:734–738.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Myasthenia Gravis Associated with Extrathymic Malignant lymphoma
- A Case of Thymic Squamous Cell Carcinoma in Myasthenia Gravis
- Pathological Findings of Tumors at Thymus and Myasthenia Gravis
- Fecal Incontinence as a Symptom of Myasthenia Gravis
- A Case of Ocular Myasthenia Gravis with Thytmolipoma and Thymic Cyst