Yonsei Med J.
2008 Aug;49(4):545-552. 10.3349/ymj.2008.49.4.545.
Is Electrical Stimulation Beneficial for Improving the Paralytic Effect of Botulinum Toxin Type A in Children with Spastic Diplegic Cerebral Palsy?
- Affiliations
-
- 1Department and Research Institute of Rehabilitation Medicine, Yonsei University College of Medicine, Seoul, Korea. pes1234@yuhs.ac
- KMID: 1793189
- DOI: http://doi.org/10.3349/ymj.2008.49.4.545
Abstract
- PURPOSE
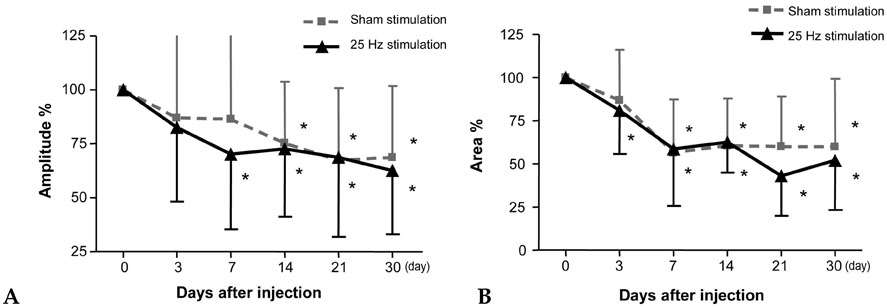
The purpose of the present study was to investigate whether electrical stimulation (ES) improves the paralytic effect of botulinum toxin type A (BTX-A) and evaluate the differences between low frequency (LF) and high frequency (HF) ES in children with spastic diplegic cerebral palsy (CP). MATERIALS and METHODS: Twenty-three children with spastic diplegia CP who had BTX-A injections into both gastrocnemius muscles were assessed. Following the toxin injection, electrical stimulation was given to 1 side of the injected muscles and a sham-stimulation to the other side for 30 min a day for 7 consecutive days [HFES (25Hz) to 11 children, LFES (4Hz) to 12 children]. The compound motor action potentials (CMAP) from the gastrocnemius muscle were assessed before injection and at 5 time points (days 3, 7, 14, 21, and 30) after injection. The clinical assessments of spasticity were performed before and 30 days after injection. RESULTS: The CMAP area became significantly lower in both LFES and HFES sides from 3 days after injection compared to baseline values. In other words, the CMAP area of the sham-stimulated side showed a significant decrease at 7 or 14 days after injection. However, there were no significant differences in clinical assessment of spasticity between the stimulated and sham-stimulated sides. CONCLUSION: Short-term ES in both LF and HF to the spastic muscles injected with BTX-A might induce earlier denervating action of BTX-A. However, it does not necessarily lead to clinical and electrophysiological benefits in terms of reduction of spasticity.
MeSH Terms
Figure
Cited by 1 articles
-
Effect of Hinged Ankle-Foot Orthoses on Standing Balance Control in Children with Bilateral Spastic Cerebral Palsy
Dong-wook Rha, Dong Jin Kim, Eun Sook Park
Yonsei Med J. 2010;51(5):746-752. doi: 10.3349/ymj.2010.51.5.746.
Reference
-
1. Mongan D, Dunne K, O'Nuallain S, Gaffney G. Prevalence of cerebral palsy in the West of Ireland 1990-1999. Dev Med Child Neurol. 2006. 48:892–895.
Article2. Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, et al. Trends in cerebral palsy among infants of very low birthweight (< 1500 g) or born prematurely (< 32 weeks) in 16 European centres: a database study. Lancet. 2007. 369:43–50.
Article3. Corry IS. Use of a motion analysis laboratory in assessing the effects of botulinum toxin in a cerebral palsy [dissertation]. 1995. Belfast (North Ireland): The Queens University;Thesis for doctor of medicine in the faculty of medicine.4. Cosgrove AP, Graham HK. Botulinum toxin A prevents the development of contractures in the hereditary spastic mouse. Dev Med Child Neurol. 1994. 36:379–385.
Article5. Dunne JW, Heye N, Dunne SL. Treatment of chronic limb spasticity with botulinum toxin A. J Neurol Neurosurg Psychiatry. 1995. 58:232–235.
Article6. Lance JW. Feldman RG, Young RR, Koella WP, editors. Pathophysiology of spasticity and clinical experience of baclofen. Spasticity: disordered motor control. 1980. Chicago: Year Book Medical Publishers;185–203.7. Ziv I, Blackburn N, Rang M, Koreska J. Muscle growth in normal and spastic mice. Dev Med Child Neurol. 1984. 26:94–99.
Article8. Mohamed KA, Moore AP, Rosenbloom L. Adverse events following repeated injections with botulinum toxin A in children with spasticity. Dev Med Child Neurol. 2001. 43:791.
Article9. Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol. 2001. 8 Suppl 5:21–29.
Article10. Eleopra R, Tugnoli V, De Grandis D. The variability in the clinical effect induced by botulinum toxin type A: the role of muscle activity in humans. Mov Disord. 1997. 12:89–94.
Article11. Hesse S, Jahnke MT, Luecke D, Mauritz KH. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett. 1995. 201:37–40.
Article12. Hughes R, Whaler BC. Influence of nerve-ending activity and of drugs on the rate of paralysis of rat diaphragm preparations by Cl. botulinum type A toxin. J Physiol. 1962. 160:221–233.
Article13. Kim HS, Hwang JH, Jeong ST, Lee YT, Lee PK, Suh YL, et al. Effect of muscle activity and botulinum toxin dilution volume on muscle paralysis. Dev Med Child Neurol. 2003. 45:200–206.
Article14. Frasson E, Priori A, Ruzzante B, Didone G, Bertolasi L. Nerve stimulation boosts botulinum toxin action in spasticity. Mov Disord. 2005. 20:624–629.
Article15. Detrembleur C, Lejeune TM, Renders A, Van Den Bergh PY. Botulinum toxin and short-term electrical stimulation in the treatment of equinus in cerebral palsy. Mov Disord. 2002. 17:162–169.
Article16. Hesse S, Reiter F, Konrad M, Jahnke MT. Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: a randomized, double-blind, placebo-controlled trial. Clin Rehabil. 1998. 12:381–388.
Article17. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997. 39:214–223.
Article18. Seo JH, Kim SS, Kim NK, Lee JH. Measuring compound muscle action potentials after botulinum toxin a injection for the quantification of effects. J Korean Acad Rehabil Med. 1998. 22:1225–1231.19. Yam WK, Leung MS. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol. 2006. 21:1031–1035.
Article20. Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos Trans R Soc Lond B Biol Sci. 1999. 354:259–268.
Article21. Das Gupta BR, Sugiyama H. Role of a protease in natural activation of Clostridium botulinum neurotoxin. Infect Immun. 1972. 6:587–590.
Article22. Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980. 212:16–21.23. Kesar T, Chou LW, Binder-Macleod SA. Effects of stimulation frequency versus pulse duration modulation on muscle fatigue. J Electromyogr Kinesiol. 2007. 18:662–671.
Article24. Kesar T, Binder-Macleod S. Effect of frequency and pulse duration on human muscle fatigue during repetitive electrical stimulation. Exp Physiol. 2006. 91:967–976.
Article25. Johnson C, Wood DE, Swain ID, Tromans AM, Strike P, Burridge JH. A pilot study to investigate the combined use of botulinum neurotoxin type a and functional electrical stimulation, with physiotherapy, in the treatment of spastic dropped foot in subacute stroke. Artif Organs. 2002. 26:263–266.
Article26. Carda S, Molteni F. Taping versus electrical stimulation after botulinum toxin type A injection for wrist and finger spasticity. A case-control study. Clin Rehabil. 2005. 19:621–626.
Article27. Rodriquez AA, McGinn M, Chappell R. Botulinum toxin injection of spastic finger flexors in hemiplegic patients. Am J Phys Med Rehabil. 2000. 79:44–47.
Article28. Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil. 1999. 13:373–383.
Article29. Love SC, Valentine JP, Blair EM, Price CJ, Cole JH, Chauvel PJ. The effect of botulinum toxin type A on the functional ability of the child with spastic hemiplegia a randomized controlled trial. Eur J Neurol. 2001. 8 Suppl 5:50–58.
Article30. Mehrholz J, Wagner K, Meissner D, Grundmann K, Zange C, Koch R, et al. Reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in adult patients with severe brain injury: a comparison study. Clin Rehabil. 2005. 19:751–759.
Article31. Sätilä H, Iisalo T, Pietikäinen T, Seppänen RL, Salo M, Koivikko M, et al. Botulinum toxin treatment of spastic equinus in cerebral palsy: a randomized trial comparing two injection sites. Am J Phys Med Rehabil. 2005. 84:355–365. quiz 66-7, 392.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of electrical stimulation on the trunk control in young children with spastic diplegic cerebral palsy
- Effects of Botulinum Toxin A Treatment in Cerebral Palsy
- The Change of Intrinsic Stiffness in Gastrocnemius after Intensive Rehabilitation with Botulinum Toxin A Injection in Spastic Diplegic Cerebral Palsy
- Electrophysiological Changes after Botulinum Toxin Type A in Children with Cerebral Palsy
- Effects of Electrical Stimulation on the Prolongation of Botulinum Toxin Type A Induced Paralysis