J Korean Med Sci.
2010 Dec;25(12):1722-1726. 10.3346/jkms.2010.25.12.1722.
In vitro Evaluation of Antibiotic Lock Technique for the Treatment of Candida albicans, C. glabrata, and C. tropicalis Biofilms
- Affiliations
-
- 1Department of Molecular Cell Biology, Sungkyunkwan University School of Medicine, Suwon, Korea.
- 2Asia Pacific Foundation for Infectious Diseases (APFID), Seoul, Korea.
- 3Division of Infectious Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. krpeck@skku.edu
- KMID: 1792894
- DOI: http://doi.org/10.3346/jkms.2010.25.12.1722
Abstract
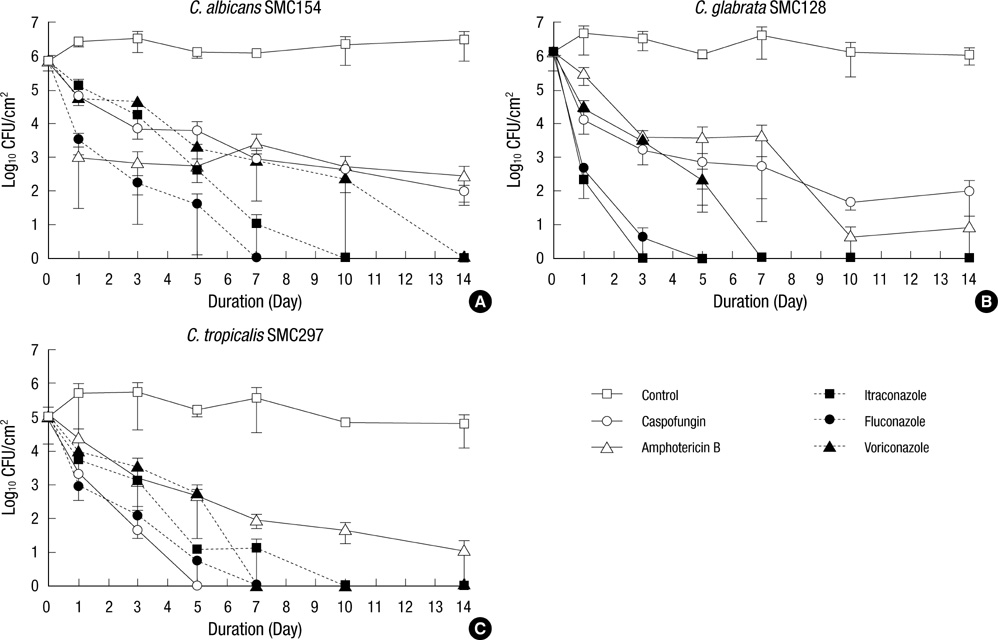
- Candidaemia associated with intravascular catheter-associated infections is of great concern due to the resulting high morbidity and mortality. The antibiotic lock technique (ALT) was previously introduced to treat catheter-associated bacterial infections without removal of catheter. So far, the efficacy of ALT against Candida infections has not been rigorously evaluated. We investigated in vitro activity of ALT against Candida biofilms formed by C. albicans, C. glabrata, and C. tropicalis using five antifungal agents (caspofungin, amphotericin B, itraconazole, fluconazole, and voriconazole). The effectiveness of antifungal treatment was assayed by monitoring viable cell counts after exposure to 1 mg/mL solutions of each antibiotic. Fluconazole, itraconazole, and voriconazole eliminated detectable viability in the biofilms of all Candida species within 7, 10, and 14 days, respectively, while caspofungin and amphotericin B did not completely kill fungi in C. albicans and C. glabrata biofilms within 14 days. For C. tropicalis biofilm, caspofungin lock achieved eradication more rapidly than amphotericin B and three azoles. Our study suggests that azoles may be useful ALT agents in the treatment of catheter-related candidemia.
Keyword
MeSH Terms
-
Amphotericin B/administration & dosage/pharmacology
Antifungal Agents/*administration & dosage/pharmacology/therapeutic use
Biofilms/*drug effects
Candida albicans/*drug effects/physiology
Candida glabrata/*drug effects/physiology
Candida tropicalis/*drug effects/physiology
Candidiasis/drug therapy
Catheter-Related Infections/drug therapy
Catheterization, Central Venous
Drug Administration Routes
Echinocandins/administration & dosage/pharmacology
Fluconazole/administration & dosage/pharmacology
Humans
Itraconazole/administration & dosage/pharmacology
Microbial Sensitivity Tests
Pyrimidines/administration & dosage/pharmacology
Triazoles/administration & dosage/pharmacology
Figure
Reference
-
1. Han SS, Yim JJ, Yoo CG, Kim YW, Han SK, Shim YS, Lee SM. Clinical characteristics and risk factors for nosocomial candidemia in medical intensive care units: experience in a single hospital in Korea for 6.6 years. J Korean Med Sci. 2010. 25:671–676.
Article2. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009. 49:1–45.
Article3. Mukherjee PK, Long L, Kim HG, Ghannoum MA. Amphotericin B lipid complex is efficacious in the treatment of Candida albicans biofilms using a model of catheter-associated Candida biofilms. Int J Antimicrob Agents. 2009. 33:149–153.
Article4. O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002. 51:1–29.5. Cateau E, Rodier MH, Imbert C. In vitro efficacies of caspofungin or micafungin catheter lock solutions on Candida albicans biofilm growth. J Antimicrob Chemother. 2008. 62:153–155.
Article6. Ramage G, VandeWalle K, Bachmann SP, Wickes BL, López-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against performed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002. 46:3634–3636.7. Shuford JA, Rouse MS, Piper KE, Steckelberg JM, Patel R. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J Infect Dis. 2006. 194:710–713.8. Lee JY, Ko KS, Peck KR, Oh WS, Song JH. In vitro evaluation of the antibiotic lock technique (ALT) for the treatment of catheter-related infections caused by staphylococci. J Antimicrob Chemother. 2006. 57:1110–1115.
Article9. Lee MY, Ko KS, Song JH, Peck KR. In vitro effectiveness of the antibiotic lock technique (ALT) for the treatment of catheter-related infections by Pseudomonas aeruginosa and Klebsiella pneumoniae. J Antimicrob Chemother. 2007. 60:782–787.
Article10. Donlan RM. Biofilms on central venous catheters: is eradication possible? Curr Top Microbiol Immunol. 2008. 322:133–161.
Article11. Clinical and Laboratory Standards Institute. Document No. M27-A2. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard. 2008. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute.12. Hawser SP, Norris H, Jessup CJ, Ghannoum MA. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998. 36:1450–1452.13. Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002. 46:1773–1780.
Article14. Golomb G, Shpigelman A. Prevention of bacterial colonization on polyurethane in vitro by incorporated antibacterial agent. J Biomed Mater Res. 1991. 25:937–952.15. Melo AS, Colombo AL, Arthington-Skaggs BA. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob Agents Chemother. 2007. 51:3081–3088.
Article16. Raad II, Hanna HA. Intravascular catheter-related infections: new horizons and recent advances. Arch Intern Med. 2002. 162:871–878.17. Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, Mohamed S, Ghannoum MA. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob Agents Chemother. 2004. 48:1727–1732.
Article18. Buckler BS, Sams RN, Goei VL, Krishnan KR, Bemis MJ, Parker DP, Murray DL. Treatment of central venous catheter fungal infection using liposomal amphotericin-B lock therapy. Pediatr Infect Dis J. 2008. 27:762–764.
Article19. Bachmann SP, Ramage G, VandeWalle K, Patterson TF, Wickes BL, López-Ribot JL. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob Agents Chemother. 2003. 47:3657–3659.
Article20. Ferreira JA, Carr JH, Starling CE, De Resende MA, Donlan RM. Biofilm formation and the effect of caspofungin on the biofilm structure of Candida species bloodstream isolates. Antimicrob Agents Chemother. 2009. 53:4377–4384.21. Perumal P, Mekala S, Chaffin WL. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob Agents Chemother. 2007. 51:2454–2463.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of Candida Species Using CHROMagar Candida in Superficial Cutaneous Candidiasis
- A Case of Candida Glabrata Infection after Total Knee Arthroplasty
- Direct Presumptive Identification of Candida species from Blood Cultures Using CHROMagar Candida
- Seroreactivities of proteinases of Candida albicans, C. tropicalis, and C. parapsilosis in sera from various Candida species-infected mice
- Risk Factors for Hospital-Acquired Urinary Tract Infection due to Candida Species