Korean J Ophthalmol.
2014 Feb;28(1):108-112. 10.3341/kjo.2014.28.1.108.
Anaplastic Large Cell Lymphoma Involving Anterior Segment of the Eye
- Affiliations
-
- 1Department of Ophthalmology, Dongguk University Seoul, Graduate School of Medicine, Seoul, Korea. blueretinaoh@gmail.com
- 2Department of Internal Medicine, Dongguk University Seoul, Graduate School of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Dongguk University Seoul, Graduate School of Medicine, Seoul, Korea.
- KMID: 1792102
- DOI: http://doi.org/10.3341/kjo.2014.28.1.108
Abstract
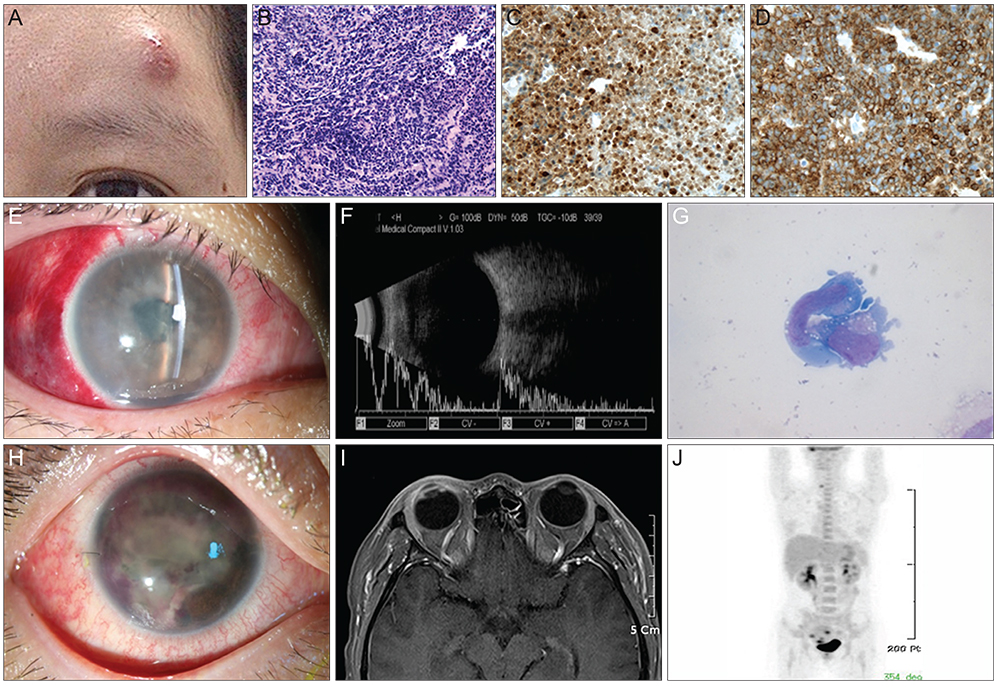
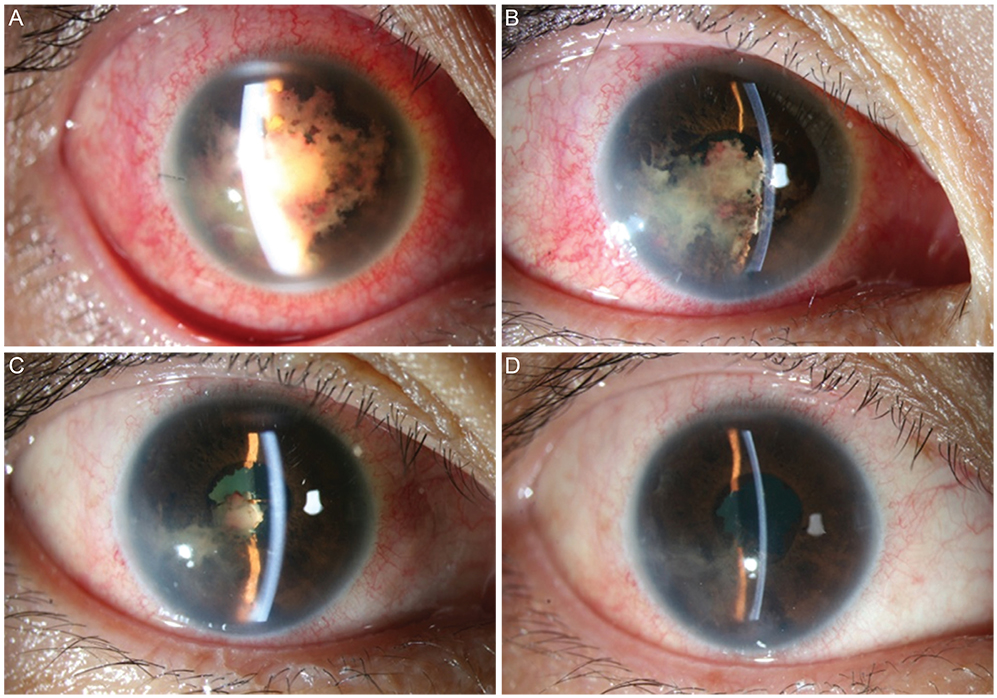
- A 36-year-old woman was diagnosed with anaplastic large cell lymphoma (ALCL) by excisional biopsy of a left frontal skin lesion. During the first cycle of chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone), the patient complained of right ocular pain and inflammation. Cytologic examination using aqueous humor revealed atypical lymphocytes, suggesting intraocular ALCL involvement. Acute angle closure developed in the anterior chamber due to rapid progression of ALCL, causing pupillary block. Laser and surgical interventions were attempted but failed to relieve the pupillary block. Finally, radiation therapy resolved the pupillary block to restore the anterior chamber and normalize intraocular pressure. This is the first case in the English literature of ALCL involving the iris to cause acute secondary angle closure.
MeSH Terms
Figure
Reference
-
1. Fornari A, Piva R, Chiarle R, et al. Anaplastic large cell lymphoma: one or more entities among T-cell lymphoma? Hematol Oncol. 2009; 27:161–170.2. Inghirami G, Pileri SA. European T-Cell Lymphoma Study Group. Anaplastic large-cell lymphoma. Semin Diagn Pathol. 2011; 28:190–201.3. Medeiros LJ, Elenitoba-Johnson KS. Anaplastic large cell lymphoma. Am J Clin Pathol. 2007; 127:707–722.4. Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Experiment Ophthalmol. 2008; 36:564–578.5. Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol. 2004; 242:901–913.6. Coupland SE, Anastassiou G, Bornfeld N, et al. Primary intraocular lymphoma of T-cell type: report of a case and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2005; 243:189–197.7. Levy-Clarke GA, Greenman D, Sieving PC, et al. Ophthalmic manifestations, cytology, immunohistochemistry, and molecular analysis of intraocular metastatic T-cell lymphoma: report of a case and review of the literature. Surv Ophthalmol. 2008; 53:285–295.8. Kohno T, Uchida H, Inomata H, et al. Ocular manifestations of adult T-cell leukemia/lymphoma. A clinicopathologic study. Ophthalmology. 1993; 100:1794–1799.9. Shibata K, Shimamoto Y, Nishimura T, et al. Ocular manifestations in adult T-cell leukemia/lymphoma. Ann Hematol. 1997; 74:163–168.10. Mashayekhi A, Shields CL, Shields JA. Iris involvement by lymphoma: a review of 13 cases. Clin Experiment Ophthalmol. 2013; 41:19–26.11. Shields CL, Shields JA, Shields MB, Augsburger JJ. Prevalence and mechanisms of secondary intraocular pressure elevation in eyes with intraocular tumors. Ophthalmology. 1987; 94:839–846.12. Kim WI, Larar JG, Cheson BD, et al. Resolution of lymphoma-associated open-angle glaucoma by rituximab. J Glaucoma. 2011; 20:398–400.13. Matsui N, Kamao T, Azumi A. Case of metastatic intraocular malignant lymphoma with neovascular glaucoma. Nihon Ganka Gakkai Zasshi. 2005; 109:434–439.14. Berthold S, Kottler UB, Frisch L, et al. Secondary glaucoma in hyphema, hypopyon, iris prominence and iris hyperemia. Ophthalmologe. 2005; 102:290–292.15. Patel AS, Hemady RK, Rodrigues M, et al. Endogenous Fusarium endophthalmitis in a patient with acute lymphocytic leukemia. Am J Ophthalmol. 1994; 117:363–368.16. Gupta SR, Agnani S, Tehrani S, et al. Endogenous Streptococcus agalactiae (Group B Streptococcus) endophthalmitis as a presenting sign of precursor T-cell lymphoblastic leukemia. Arch Ophthalmol. 2010; 128:384–385.17. Dave VP, Majji AB, Suma N, Pappuru RR. A rare case of Aspergillus terreus endogenous endophthalmitis in a patient of acute lymphoid leukemia with good clinical outcome. Eye (Lond). 2011; 25:1094–1096.18. Sen HN, Bodaghi B, Hoang PL, Nussenblatt R. Primary intraocular lymphoma: diagnosis and differential diagnosis. Ocul Immunol Inflamm. 2009; 17:133–141.19. Drancourt M, Raoult D, Lepidi H, et al. Culture of Tropheryma whippelii from the vitreous fluid of a patient presenting with unilateral uveitis. Ann Intern Med. 2003; 139:1046–1047.20. Finger PT, Papp C, Latkany P, et al. Anterior chamber paracentesis cytology (cytospin technique) for the diagnosis of intraocular lymphoma. Br J Ophthalmol. 2006; 90:690–692.21. Matsuo T, Ichimura K, Ichikawa T, et al. Positron emission tomography/computed tomography after immunocytochemical and clonal diagnosis of intraocular lymphoma with vitrectomy cell blocks. J Clin Exp Hematop. 2009; 49:77–87.22. Margolis L, Fraser R, Lichter A, Char DH. The role of radiation therapy in the management of ocular reticulum cell sarcoma. Cancer. 1980; 45:688–692.23. Jahnke K, Thiel E, Bechrakis NE, et al. Ifosfamide or trofosfamide in patients with intraocular lymphoma. J Neurooncol. 2009; 93:213–217.24. De Smet MD, Stark-Vancs V, Kohler DR, et al. Intraocular levels of methotrexate after intravenous administration. Am J Ophthalmol. 1996; 121:442–444.25. Batchelor TT, Kolak G, Ciordia R, et al. High-dose methotrexate for intraocular lymphoma. Clin Cancer Res. 2003; 9:711–715.26. De Smet MD, Vancs VS, Kohler D, et al. Intravitreal chemotherapy for the treatment of recurrent intraocular lymphoma. Br J Ophthalmol. 1999; 83:448–451.27. Tempescul A, Pradier O, Marianowski-Cochard C, et al. Combined therapy associating systemic platinum-based chemotherapy and local radiotherapy into the treatment of primary intraocular lymphoma. Ann Hematol. 2011; 90:1117–1118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dermatofibroma in Patient with Relapsing Primary Cutaneous Anaplastic Large Cell Lymphoma
- Immunohistochemical Study for Ki-1 and EMA Antigens in Large Cell Lymphoma including Anaplastic Large Cell Lymphoma
- A Case of ALK-Negative Systemic Anaplastic Large Cell Lymphoma
- CD30-Positive Anaplastic Lymphoma Kinase-Negative Systemic Anaplastic Large-Cell Lymphoma in a 9-Year-Old Boy
- A Case of Primary Cutaneous Anaplastic Large Cell Lymphoma on the Dorsum of the Hand