Risk Management in the Clinical Laboratory
- Affiliations
-
- 1Department of Pathology, Microbiology, and Immunology, Vanderbilt University School of Medicine, Nashville, TN, USA. james.h.nichols@vanderbilt.edu
- KMID: 1791934
- DOI: http://doi.org/10.3343/alm.2014.34.4.274
Abstract
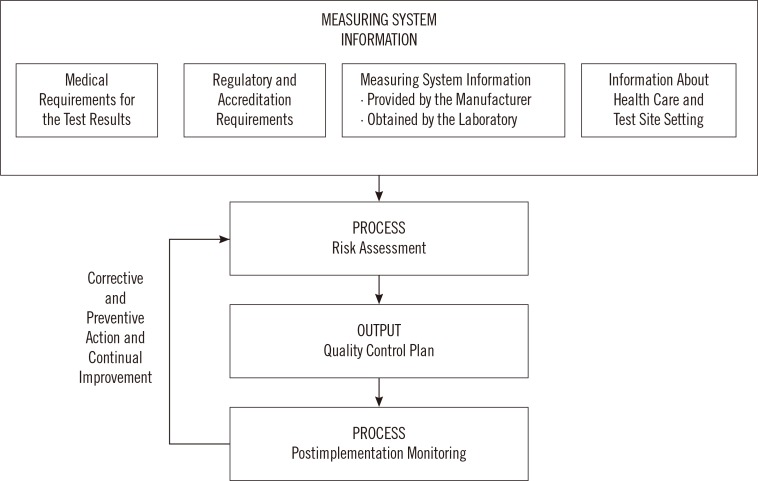
- Clinical laboratory tests play an integral role in medical decision-making and as such must be reliable and accurate. Unfortunately, no laboratory tests or devices are foolproof and errors can occur at pre-analytical, analytical and post-analytical phases of testing. Evaluating possible conditions that could lead to errors and outlining the necessary steps to detect and prevent errors before they cause patient harm is therefore an important part of laboratory testing. This can be achieved through the practice of risk management. EP23-A is a new guideline from the CLSI that introduces risk management principles to the clinical laboratory. This guideline borrows concepts from the manufacturing industry and encourages laboratories to develop risk management plans that address the specific risks inherent to each lab. Once the risks have been identified, the laboratory must implement control processes and continuously monitor and modify them to make certain that risk is maintained at a clinically acceptable level. This review summarizes the principles of risk management in the clinical laboratory and describes various quality control activities employed by the laboratory to achieve the goal of reporting valid, accurate and reliable test results.
Keyword
Figure
Cited by 6 articles
-
Clinical Pharmacogenetic Testing and Application: Laboratory Medicine Clinical Practice Guidelines Part 1
Sollip Kim, Yeo-Min Yun, In-Suk Kim, Sang Hoon Song, Hye In Woo, Kyung-A Lee, Woochang Lee, Hyun Jung Cho, Misuk Ji, Hyo-Jin Chae, Soo-Youn Lee, Sail Chun
Lab Med Online. 2016;6(3):119-133. doi: 10.3343/lmo.2016.6.3.119.Economic Evaluation of Total Laboratory Automation in the Clinical Laboratory of a Tertiary Care Hospital
KyungYi Kim, Sang-Guk Lee, Tae Hyun Kim, Sang Gyu Lee
Ann Lab Med. 2022;42(1):89-95. doi: 10.3343/alm.2022.42.1.89.Establishing Quality Control Ranges for Antimicrobial Susceptibility Testing of
Escherichia coli ,Pseudomonas aeruginosa , andStaphylococcus aureus : A Cornerstone to Develop Reference Strains for Korean Clinical Microbiology Laboratories
Sung Kuk Hong, Seung Jun Choi, Saeam Shin, Wonmok Lee, Naina Pinto, Nari Shin, Kwangjun Lee, Seong Geun Hong, Young Ah Kim, Hyukmin Lee, Heejung Kim, Wonkeun Song, Sun Hwa Lee, Dongeun Yong, Kyungwon Lee, Yunsop Chong
Ann Lab Med. 2015;35(6):635-638. doi: 10.3343/alm.2015.35.6.635.Digital Morphology Analyzer Sysmex DI-60 vs. Manual Counting for White Blood Cell Differentials in Leukopenic Samples: A Comparative Assessment of Risk and Turnaround Time
Minjeong Nam, Sumi Yoon, Mina Hur, Gun Hyuk Lee, Hanah Kim, Mikyoung Park, Hyeong Nyeon Kim
Ann Lab Med. 2022;42(4):398-405. doi: 10.3343/alm.2022.42.4.398.Review of the Use of Liquid Chromatography-Tandem Mass Spectrometry in Clinical Laboratories: Part II–Operations
Brian A. Rappold
Ann Lab Med. 2022;42(5):531-557. doi: 10.3343/alm.2022.42.5.531.Risk Analysis in HLA Typing Based on Failure Mode and Effects Analysis and Grey Relational Analysis
Jae Hyun Cha, Eunji Shin, Mina Hur, Hanah Kim, Seung Gyu Yun, Myung-Hyun Nam, Yunjung Cho, Minjeong Nam
Lab Med Online. 2025;15(2):146-153. doi: 10.47429/lmo.2025.15.2.146.
Reference
-
1. International Organization for Standardization. Medical devices - Application of risk management to medical devices ISO 14971: 2007. Geneva: International Organization for Standardization;2007.2. Clinical and Laboratory Standards Institute. Risk management techniques to identify and control laboratory error sources. Approved guideline-2nd ed. EP18-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2009.3. Clinical and Laboratory Standards Institute. Laboratory quality control based on risk management. Approved guideline-1st ed. EP23-A. Wayne, PA: Clinical and Laboratory Standards Institute;2011.4. ISO/IEC. Safety aspects - Guidelines for their inclusion in standards. ISO/IEC Guide 51. Geneva: International Organization for Standardization;1999.5. Westgard JO, Barry PL, Hunt MR, Groth T. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem. 1981; 27:493–501. PMID: 7471403.
Article6. Clinical and Laboratory Standards Institute. Statistical quality control for quantitative measurements procedures: principles and definitions. Approved guideline-3rd ed. C24-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2006.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Risk Management Examples for Korean Clinical Laboratories
- Biosafety Risk Control Strategies in Laboratory Animal Research
- International Organization for Standardization (ISO) 15189
- National Survey on Biosafety in Clinical Tuberculosis Laboratories in Korea
- Managing the Pre- and Post-analytical Phases of the Total Testing Process