Yonsei Med J.
2007 Dec;48(6):949-954. 10.3349/ymj.2007.48.6.949.
IGF-1 Counteracts TGF-beta-Mediated Enhancement of Fibronectin for in Vitro Human Lens Epithelial Cells
- Affiliations
-
- 1Department of Ophthalmology, Inje University College of Medicine, Seoul Paik Hospital, Seoul, Korea.
- 2Myunggok Eye Research Institute at Kim's Eye Hospital, Konyang University College of Medicine, Nonsan, Korea. joonhlee@konyang.ac.kr
- 3Department of Ophthalmology, Institute of Vision Research, Cornea Dystrophy Research Institute, Yonsei University College of Medicine, Seoul, Korea. eungkkim@yuhs.ac
- 4BK21 Project Team of Nanobiomaterials for Cell-based Implants, Yonsei University, Seoul, Korea.
- KMID: 1786208
- DOI: http://doi.org/10.3349/ymj.2007.48.6.949
Abstract
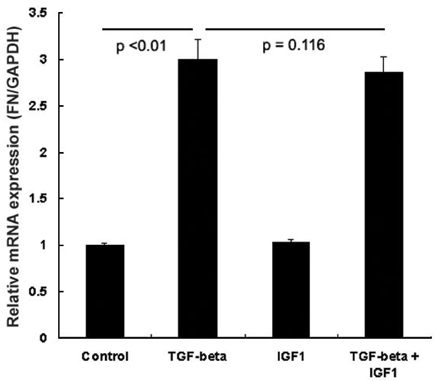
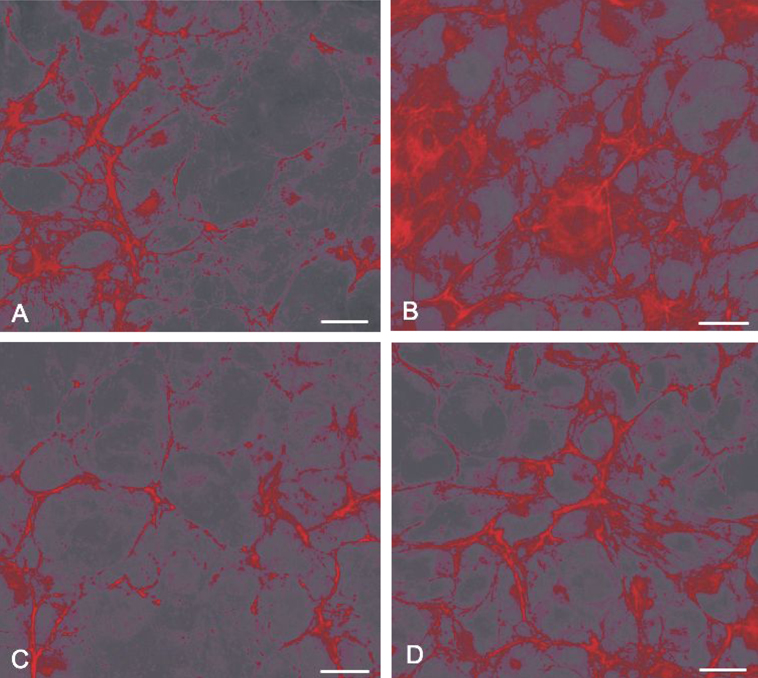
- PURPOSE: To determine whether insulin-like growth factor (IGF-1) affects transforming growth factor (TGF-beta)- mediated fibronectin accumulation in human lens epithelial cell line (HLE B-3) cells. MATERIALS AND METHODS: HLE B-3 cells were incubated for 24 hours with TGF-beta (10ng/ mL), IGF-1 (10ng/mL), or both. Expression of the fibronectin gene was determined using a real-time reverse transcriptase-polymerase chain reaction (RT-PCR). Fibronectin levels were examined using western blot analysis and immunofluorescence staining. RESULTS: Expression of the fibronectin gene was not different between the TGF-beta/IGF-1 treated group and the TGF-beta treated group (p= 0.116). However, western blot analysis demonstrated decreased fibronectin levels in human lens epithelial cells treated with TGF-beta and IGF-1 compared to those treated with TGF-beta only (p < 0.01). Immunofluorescence staining disclosed inhibition of TGF-beta-induced fibronectin in the presence of IGF-1. CONCLUSION: This study suggests that IGF-1 counteracts TGF-beta-mediated fibronectin accumulation in human lens epithelial cells.
Keyword
MeSH Terms
-
Cell Line
Epithelial Cells/cytology/*drug effects/metabolism
Fibronectins/*genetics/metabolism
Fluorescent Antibody Technique
Gene Expression Regulation/drug effects
Humans
Insulin-Like Growth Factor I/*pharmacology
Lens, Crystalline/cytology
Reverse Transcriptase Polymerase Chain Reaction
Transforming Growth Factor beta/*pharmacology
Figure
Reference
-
1. Hales AM, Schulz MW, Chamberlain CG, McAvoy JW. TGF-beta1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994. 13:885–890.
Article2. Hales AM, Chamberlain CG, McAvoy JW. Cataract induction in lenses cultured with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1995. 36:1709–1713.3. Lee EH, Joo CK. Role of transforming growth factor-beta in transdifferentiation and fibrosis of lens epithelial cells. Invest Ophthalmol Vis Sci. 1999. 40:2025–2032.4. Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, et al. TGF-beta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol. 2002. 86:220–226.
Article5. Cobo LM, Ohsawa E, Chandler D, Arguello R, George G. Pathogenesis of capsular opacification after extracapsular cataract extraction: An animal model. Ophthalmology. 1984. 91:857–863.
Article6. Lang RA. Which factors stimulate lens fiber cell differentiation in vivo? Invest Ophthalmol Vis Sci. 1999. 40:3075–3078.7. Klok E, Lubsen NH, Chamberlain CG, McAvoy JW. Induction and maintenance of differentiation of rat lens epithelium by FGF-2, insulin and IGF-1. Exp Eye Res. 1998. 67:425–431.
Article8. Andley UP, Rhim JS, Chylack LT Jr, Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest Ophthalmol Vis Sci. 1994. 35:3094–3102.9. Shin SY, Lee HJ, Ko DS, Lee HC, Park WI. The regulators of VEGF expression in mouse ovaries. Yonsei Med J. 2005. 46:679–686.
Article10. Chung SH, Lee SK, Cristol SM, Lee ES, Lee DW, Seo KY, et al. Impact of short-term exposure of commercial eyedrops preserved with benzalkonium chloride on precorneal mucin. Mol Vis. 2006. 12:415–421.11. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001. 25:402–408.
Article12. Novotny GEK, Pau H. Myofibroblast-like cells in human anterior capsular cataract. Virchows Arch. 1984. 404:393–401.
Article13. Hatae T, Ishibashi T, Yoshitomi F, Shibata Y. Immunocytochemistry of types I-IV collagen in human anterior subcapsular cataracts. Graefes Arch Clin Exp Ophthalmol. 1993. 231:586–590.
Article14. Font RL, Brownstein S. A light and electron microscopic study of anterior subcapsular cataracts. Am J Ophthalmol. 1974. 78:972–984.
Article15. de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005. 179:43–55.
Article16. Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest. 1998. 101:625–634.
Article17. Zuk A, Hay ED. Expression of beta1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev Dyn. 1994. 201:378–393.
Article18. Daian T, Ohtsuru A, Rogounovitch T, Ishihara H, Hirano A, Akiyama-Uchida Y, et al. Insulin-like growth factor-I enhances transforming growth factor-beta-induced extracellular matrix protein production through the P38/activating transcription factor-2 signaling pathway in keloid fibroblasts. J Invest Dermatol. 2003. 120:956–962.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The changes of fibronectin in the cultured porcine lens epithelial cells
- Effects of TGF-beta onthe HPC-16-induced Neoplastic Transformation of Human Cells
- Asian Dust Particles Induce TGF-beta1 via Reactive Oxygen Species in Bronchial Epithelial Cells
- Particulate Matter 10 from Asian Dust Storms Induces the Expression of Reactive Oxygen Species, NF-kappaB, TGF-beta and Fibronectin in WI-26 VA4 Epithelial Cells
- The Expression of TGF-beta Isoform mRNA in the Cataract Lens Epithelial Cell