J Korean Med Assoc.
2012 Apr;55(4):371-378. 10.5124/jkma.2012.55.4.371.
Trends in the development of and emerging tasks of clinical practice guidelines in Korea
- Affiliations
-
- 1Department of Preventive Medicine & Public Health, Ajou University School of Medicine, Suwon, Korea. shin2738@ajou.ac.kr
- KMID: 1785372
- DOI: http://doi.org/10.5124/jkma.2012.55.4.371
Abstract
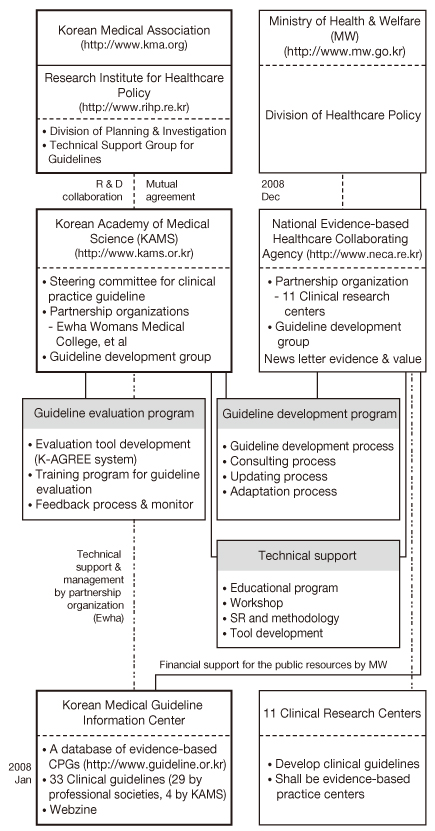
- Clinical practice guidelines (CPGs) have been developed by the members of the Korean Academy of Medical Science (KAMS) under the limited financial support of the Ministry of Health & Welfare and partnership organizations including the 11 Clinical Research Centers of the National Evidence-based Healthcare Collaborating Agency (NECA) in Korea. The Korean Medical Guideline Information Center (KoMGi), a database of evidence-based CPGs, opened on January, 2008, and it is a very useful nationwide dissemination tool of CPGs for health professionals. The following is a summary of emerging tasks related to CPGs in Korea. First, a strategy for efficient partnership between the private sector and government should be formulated and executed. Second, budget preparation and planning of financial support is needed for developing and disseminating evidence-based CPGs. Third, development of Korean-specific methodology is needed and technical support should be provided. Fourth, an educational training program should be developed for core confidence of guideline development groups. Fifth, to share current information and accelerate the dissemination of CPGs, the functions of Guideline Information Center should be reorganized and utilized. To develop highly applicable evidence-based CPGs, multidisciplinary communication and active participation among professional societies is desired.
MeSH Terms
Figure
Cited by 1 articles
-
Performance of Evidence-based Pain Assessment and Management Guidelines among Medical-Surgical Nurses
Heui Lyang Kim, Chi Eun Song, Hyang Sook So
Korean J Adult Nurs. 2016;28(5):546-558. doi: 10.7469/kjan.2016.28.5.546.
Reference
-
1. Field MJ, Lohr KN. Clinical practice guidelines: directions for a new program. 1990. Washington, DC: National Academy Press.2. Ministry of Health & Welfare; Korean Academy of Medical Science. A model for development policy of clinical practice guidelines and development of some standardized guidelines. 2007. Seoul: Ministry of Health & Welfare.3. Clinical practice guidelines: weakness of government leading. The Korean Doctors' Weekly. 2007. 01. 22.4. Grimshaw J, Russell I. Achieving health gain through clinical guidelines. I. Developing scientifically valid guidelines. Qual Health Care. 1993. 2:243–248.
Article5. Eddy DM. American College of Physicians. A manual for assessing health practices and designing practice policies: guidelines for the development and implementation of clinical practice guidelines: the explicit approach. 1992. Philadelphia: American College of Physicians;126.6. Field MJ, Lohr KN. Institute of Medicine (U.S.). Committee to Advise the Public Health Service on Clinical Practice Guidelines. United States Department of Health and Human Services. Clinical practice guidelines: directions for a new program. 1990. Washington, DC: National Academy Press;160.7. Ministry of Health & Welfare. Korean Academy of Medical Science. A study of dissemination of Clinical Practice Guidelines and utilization of guideline information center. Seoul: Ministry of Health & Welfare.8. Burgers JS, Grol R, Klazinga NS, Makela M, Zaat J. AGREE Collaboration. Towards evidence-based clinical practice: an international survey of 18 clinical guideline programs. Int J Qual Health Care. 2003. 15:31–45.
Article9. Alonso-Coello P, Irfan A, Sola I, Gich I, Delgado-Noguera M, Rigau D, Tort S, Bonfill X, Burgers J, Schunemann H. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010. 19:e58.
Article10. Guideline International Network. About G-I-N [Internet]. c2002-2012. cited 2012 Feb 1. Berlin: G-I-N office;Available from: http://www.g-i-n.net/about-g-i-n.11. National Guideline Clearinghouse. Guidelines by topic [Internet]. cited 2012 Feb 1. Rockville: Agency for Healthcare Research and Quality;Available from: http://guideline.gov/browse/by-topic.aspx.12. National Institute for Health and Clinical Excellence. Guidence by type [Internet]. c2012. cited 2012 Feb 1. London: National Institute for Health and Clinical Excellence;Available from: http://www.nice.org.uk/guidance/index.jsp?action=byType.13. Korean Medical Guideline Information Center. About us [Internet]. c2008. cited 2012 Feb 1. Seoul: Korean Medical Guideline Information Center;Available from: http://www.guideline.or.kr/contents/index.php?code=044.14. Ministry of Health & Welfare. Korean Academy of Medical Science. Adaptation process for developing Korean clinical practice guidelines. 2011. Seoul: Ministry of Health & Welfare.15. Ministry of Health & Welfare. Korean Academy of Medical Science. A model for development, education, dissemination of Korean Clinical Practice Guideline and web-based guideline evaluation system using AGREE 2.0 instrument. 2011. Seoul: Ministry of Health & Welfare.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and Implementation of Clinical Practice Guidelines: Current Status in Korea
- Intention to Delegate Clinical Practice of Medical Specialists in Accordance with the Enactment of the Scope of Practice for Advanced Practice Nurses
- Job Performance of Advanced Practice Nurses, Perceived Difficulty and Importance, and Willingness to Legally Delegate Clinical Practices to Advanced Practice Nurses by Health Care Professionals
- Methodology of revision of Korean national cancer screening guideline
- Nurses' Usage of Clinical Practice Guideline and Demand of Evidence Based Clinical Practice Guideline