J Vet Sci.
2014 Jun;15(2):273-281. 10.4142/jvs.2014.15.2.273.
Effect of superovulation on uterine and serum biochemical parameters and its potential association with transferable embryos in Holstein dairy cows
- Affiliations
-
- 1Departement of Veterinary Biomedicine, Faculty of Veterinary Medicine, University of Montreal, Saint-Hyacinthe J2S 7C6, Canada. younes.chorfi@umontreal.ca
- 2Medi-Vet Inc., Notre-Dame-du-Bon-Conseil J0C 1A0, Canada.
- 3Animal Reproduction Research Center (CRRA), Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Montreal, Saint-Hyacinthe J2S 7C6, Canada.
- KMID: 1784649
- DOI: http://doi.org/10.4142/jvs.2014.15.2.273
Abstract
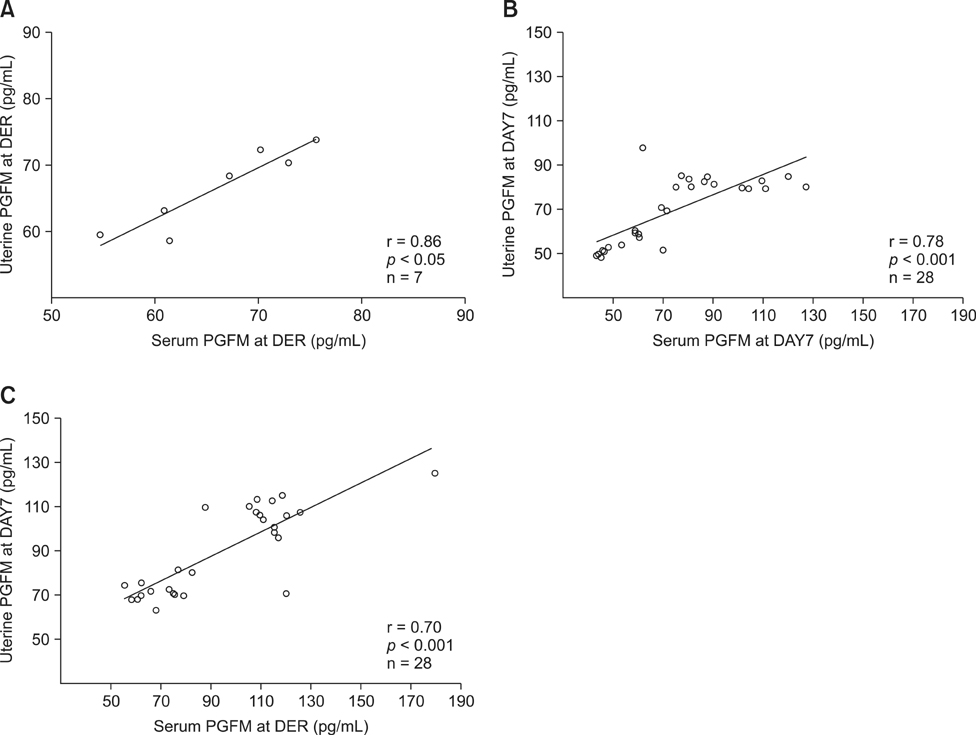
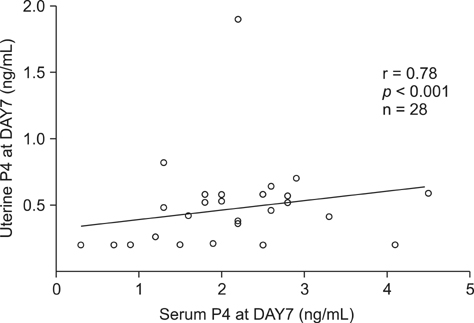
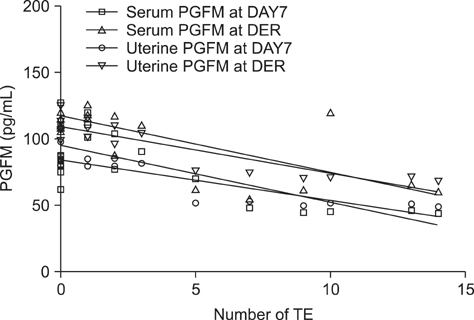
- The objective of this study was to determine the effects of superovulation (SOV) on serum and uterine biochemical parameters, uterine bacteriology and cytology and number of transferable embryos (TE). Dairy cows were placed on a Presynch/CIDR Synch protocol. The SOV group was superovulated, induced in estrus, and inseminated, whereas the control group was induced in estrus and inseminated without SOV. Uterine bacteriology and cytology and uterine and serum biochemical parameters were measured at day 7 of the estrous cycle to start the SOV protocol, as well as on the day of embryo recovery (DER). The SOV group produced 7.5 +/- 6.7 oocytes/embryos, of which 3.4 +/- 4.7 were TE. Serum urea and E2 and uterine Glu, CK, LDH, TP, P4 and PGFM in the control group and serum P4 and PGFM and uterine LDH and PGFM in the SOV group were significantly higher (p < 0.01) at DER than day 7. At DER, uterine urea, LDH, PGFM and TP and serum urea, LDH, PGFM, and P4 concentrations were higher (p < 0.01) in the SOV group than the control. There was no significant variation in uterine bacteriology or cytology. Overall, these results infer that SOV affects both serum profile and uterine secretions, and that these changes may influence the number of TE.
MeSH Terms
Figure
Reference
-
1. Bavister BD. Interactions between embryos and the culture milieu. Theriogenology. 2000; 53:619–626.
Article2. Bényei B, Gáspárdy A, Komlósi I, Pécsi A. Repeatability and heritability of ovulation number and embryos in dam-daughters pairs in superovulated Holstein-Friesian cows. Reprod Domest Anim. 2004; 39:99–102.
Article3. Chorfi Y, Lanevaschi A, Dupras R, Girard V, Tremblay A. Serum biochemical parameters and embryo production during superovulatory treatment in dairy cattle. Res Vet Sci. 2007; 83:318–321.
Article4. Cushman RA, DeSouza JC, Hedgpeth VS, Britt JH. Superovulatory response of one ovary is related to the microand macroscopic population of follicles in the contralateral ovary of the cow. Biol Reprod. 1999; 60:349–354.
Article5. Davis AJ, Fleet IR, Hansford PA, Harrison FA, Maule Walker FM. Pulmonary metabolism of prostaglandin F2α in the conscious non-pregnant cow. J Physiol. 1985; 358:Suppl. 107.6. Dos Santos RM, Goissis MD, Fantini DA, Bertan CM, Vasconcelos JLM, Binelli M. Elevated progesterone concentrations enhance prostaglandin F2α synthesis in dairy cows. Anim Reprod Sci. 2009; 114:62–71.
Article7. Drillich M, Tesfaye D, Rings F, Schellander K, Heuwieser W, Hoelker M. Effects of polymorphonuclear neutrophile infiltration into the endometrial environment on embryonic development in superovulated cows. Theriogenology. 2012; 77:570–578.
Article8. Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Definition and diagnosis of postpartum endometritis in dairy cows. J Dairy Sci. 2010; 93:5225–5233.9. Edmonson AJ, Lean IJ, Weaver LD, Farver T, Webster G. A body condition scoring chart for Holstein dairy cows. J Dairy Sci. 1989; 72:68–78.
Article10. Gardner DK, Lane M. Development of viable mammalian embryos in vitro: evolution of sequential media. In : Cibelli J, Lanza R, Campbell KHS, West MD, editors. Principles of Cloning. 1st ed. Amsterdam: Academic Press;2002. p. 187–213.11. Gibbons J, Hewitt E, Gardner DK. Effects of oxygen tension on the establishment and lactate dehydrogenase activity of murine embryonic stems cells. Cloning Stem Cells. 2006; 8:117–122.
Article12. Hockett ME, Hopkins FM, Lewis MJ, Saxton AM, Dowlen HH, Oliver SP, Schrick FN. Endocrine profiles of dairy cows following experimentally induced clinical mastitis during early lactation. Anim Reprod Sci. 2000; 58:241–251.
Article13. Hugentobler SA, Humpherson PG, Leese HJ, Sreenan JM, Morris DG. Energy substrates in bovine oviduct and uterine fluid and blood plasma during the oestrous cycle. Mol Reprod Dev. 2008; 75:496–503.
Article14. Hwang DH, Pool SH, Rorie RW, Boudreau M, Godke RA. Transitional changes in arachidonic acid metabolism by bovine embryos at different developmental stages. Prostaglandins. 1988; 35:387–402.
Article15. Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH. A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can Vet J. 2005; 46:255–259.16. Killian GJ. Evidence for the role of oviduct secretions in sperm function, fertilization and embryo development. Anim Reprod Sci. 2004; 82-83:141–153.
Article17. Kim IH, Son DS, Yeon SH, Choi SH, Park SB, Ryu IS, Suh GH, Lee DW, Lee CS, Lee HJ, Yoon JT. Effect of dominant follicle removal before superstimulation on follicular growth, ovulation and embryo production in Holstein cows. Theriogenology. 2001; 55:937–945.
Article18. Kimura K, Spate LD, Green MP, Roberts RM. Effects of D-glucose concentration, D-fructose, and inhibitors of enzymes of the pentose phosphate pathway on the development and sex ratio of bovine blastocysts. Mol Reprod Dev. 2005; 72:201–207.
Article19. Lonergan P, Woods A, Fair T, Carter F, Rizos D, Ward F, Quinn K, Evans A. Effect of embryo source and recipient progesterone environment on embryo development in cattle. Reprod Fertil Dev. 2007; 19:861–868.
Article20. Mapletoft RJ, Steward KB, Adams GP. Recent advances in the superovulation in cattle. Reprod Nutr Dev. 2002; 42:601–611.
Article21. Matsuyama K, Miyakoshi H, Fukui Y. Effect of glucose levels during the in vitro culture in synthetic oviduct fluid medium on in vitro development of bovine oocytes matured and fertilized in vitro. Theriogenology. 1993; 40:595–605.
Article22. McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev. 1999; 79:263–323.
Article23. Noakes DE, Wallace L, Smith GR. Bacterial flora of the uterus of cows after calving on two hygienically contrasting farms. Vet Rec. 1991; 128:440–442.
Article24. Okumu LA, Forde N, Fahey AG, Fitzpatrick E, Roche JF, Crowe MA, Lonergan P. The effect of elevated progesterone and pregnancy status on mRNA expression and localisation of progesterone and oestrogen receptors in the bovine uterus. Reproduction. 2010; 140:143–153.
Article25. Olfert ED, Cross BM, McWilliam AA, editors. Guide to the Care and Use of Experimental Animals. Vol. 1. Ottawa: Canadian Council on Animal Care;1993. p. 212.26. Peter AT, Bosu WTK. Peripartal endocrine changes associated with retained placenta in dairy cows. Theriogenology. 1987; 28:383–394.
Article27. Rhoads ML, Rhoads RP, Gilbert RO, Toole R, Butler WR. Detrimental effects of high plasma urea nitrogen levels on viability of embryos from lactating dairy cows. Anim Reprod Sci. 2006; 91:1–10.
Article28. Robertson I, Nelson RE. Certification and identification of embryos. In : Stringfellow DA, Seidel SM, editors. Manual of the International Embryo Transfer Society. 3rd ed. Savoy: IETS;2000. p. 109–123.29. Sangsritavong S, Coombs DK, Sartori R, Armentano LE, Wiltbank MC. High feed intake increases blood flow and metabolism of progesterone and estradiol-17β in dairy cows. J Dairy Sci. 2002; 85:2831–2842.
Article30. Santos JEP, Cerri RLA, Sartori R. Nutritional management of the donor cow. Theriogenology. 2008; 69:88–97.
Article31. Sartori R, Suárez-Fernández CA, Monson RL, Guenther JN, Rosa GJM, Wiltbank MC. Improvement in recovery of embryos/ova using a shallow uterine horn flushing technique in superovulated Holstein heifers. Theriogenology. 2003; 60:1319–1330.
Article32. Scenna FN, Edwards JL, Rohrbach NR, Hockett ME, Saxton AM, Schrick FN. Detrimental effects of prostaglandin F2α on preimplantation bovine embryos. Prostaglandins Other Lipid Mediat. 2004; 73:215–226.
Article33. Schillo KK. Reproductive Physiology of Mammals: From Farm to Field and Beyond. Clifton Park: Delmar Cengage Learning;2009. p. 50–61.34. Schrick FN, Inskeep EK, Butcher RL. Pregnancy rates for embryos transferred from early postpartum beef cows into recipients with normal estrous cycles. Biol Reprod. 1993; 49:617–621.35. Singh J, Domínguez M, Jaiswal R, Adams GP. A simple ultrasound test to predict the superstimulatory response in cattle. Theriogenology. 2004; 62:227–243.
Article36. Thatcher WW, Macmillan KL, Hansen PJ, Drost M. Concepts for regulation of corpus luteum function by the conceptus and ovarian follicles to improve fertility. Theriogenology. 1989; 31:149–164.
Article37. Velez JS, Randel RD, Neuendorff DA. Effect of uterine manipulation on postpartum fertility and plasma 13,14-dihydro-15-keto-prostaglandin F2α in Brahman cows and first-calf heifers. Theriogenology. 1991; 36:987–998.
Article38. Violette MI, Madan P. Na+/K+-ATPase regulates tight junction formation and function during mouse preimplantation development. Dev Biol. 2006; 289:406–419.
Article39. Watson AJ, Barcroft LC. Regulation of blastocyst formation. Front Biosci. 2001; 6:D708–D730.
Article40. Williams EJ, Fischer DP, Noakes DE, England GCW, Rycroft A, Dobson H, Sheldon IM. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology. 2007; 68:549–559.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidence of hypocalcemia and its changes of biochemical parameters in periparturient cows
- Ultrasonographic ovarian dynamic, plasma progesterone, and non-esterified fatty acids in lame postpartum dairy cows
- Risk factors for repeat breeder dairy cows and their impacts on reproductive performance
- Latitude and seasons influence the prevalence of Theileria orientalis and affect the hematology of non-grazed dairy cows in Korea
- Effects of chelated Zn/Cu/Mn on redox status, immune responses and hoof health in lactating Holstein cows