Korean J Radiol.
2005 Jun;6(2):75-81. 10.3348/kjr.2005.6.2.75.
Assessment of Tissue Viability Using Diffusion- and Perfusion-Weighted MRI in Hyperacute Stroke
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea. wonjin.moon@samsung.com
- 2Department of Radiology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Korea.
- 3Department of Radiology, Seoul National University Hospital, Korea.
- 4Department of Radiology, Gyeongsang National University College of Medicine, Korea.
- KMID: 1783179
- DOI: http://doi.org/10.3348/kjr.2005.6.2.75
Abstract
OBJECTIVE
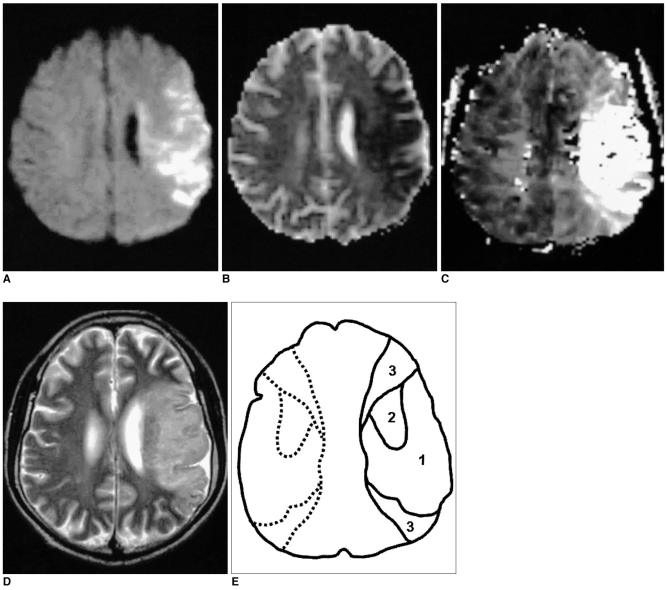
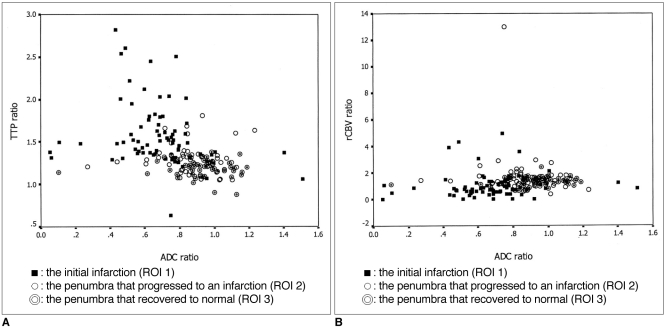
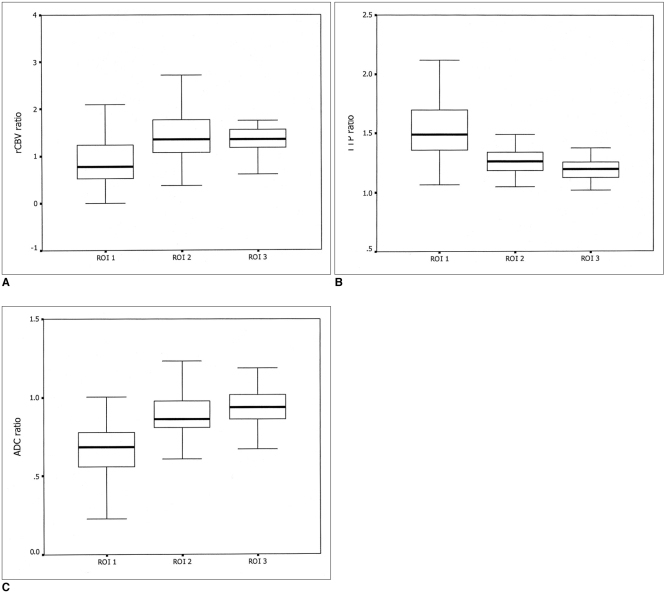
The aim of this study was to investigate the relationship between the diffusion and perfusion parameters in hyperacute infarction, and we wanted to determine the viability threshold for the ischemic penumbra using diffusion- and perfusion-weighted imaging (DWI and PWI, respectively). MATERIALS AND METHODS: Both DWI and PWI were performed within six hours from the onset of symptoms for 12 patients who had suffered from acute stroke. Three regions of interest (ROIs) were identified: ROI 1 was the initial lesion on DWI; ROI 2 was the DWI/PWI mismatch area (the penumbra) that progressed onward to the infarct; and ROI 3 was the mismatch area that recovered to normal on the follow-up scans. The ratios of apparent diffusion coefficient (ADC), the relative cerebral blood volume (rCBV), and the time to peak (TTP) were calculated as the lesions' ROIs divided by the contralateral mirror ROIs, and these values were then correlated with each other. The viability threshold was determined by using the receiver operating characteristic (ROC) curves. RESULTS: For all three ROIs, the ADC ratios had significant linear correlation with the TTP ratios (p < 0.001), but not with the rCBV ratios (p = 0.280). There was no significant difference for the ADC and rCBV ratios within the ROIs. The mean TTP ratio/TTP delay between the penumbras' two ROIs showed a significant statistical difference (p < 0.001). The cutoff value between ROI 2 and ROI 3, as the viability threshold, was a TTP ratio of 1.29 (with a sensitivity and specificity of 86% and 73%, respectively) and a TTP delay of 7.8 sec (with a sensitivity and specificity of 84% and 72%, respectively). CONCLUSION: Determining the viability thresholds for the TTP ratio/delay on the PWI may be helpful for selecting those patients who would benefit from the various therapeutic interventions that can be used during the acute phase of ischemic stroke.
Keyword
MeSH Terms
Figure
Reference
-
1. Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998; 51:418–426. PMID: 9710013.
Article2. Lovblad KO, Laubach HJ, Baird AE, Curtin F, Schlaug G, Edelman RR, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol. 1998; 19:1061–1066. PMID: 9672012.3. Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and pergusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab. 1996; 16:53–59. PMID: 8530555.4. Schlaug G, Benfield A, Baird AE, Siewert B, Lovbald KO, Parker PA, et al. The ischemic penumbra operationally defined by diffusion and perfusion MRI. Neurology. 1999; 53:1528–1537. PMID: 10534263.
Article5. Sorensen AG, Copen WA, Ostergaard L, Buonanno FS, Gonzales RG, Rordorf G, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology. 1999; 210:519–527. PMID: 10207439.
Article6. Neumann-Haefelin T, Wittsack HJ, Wenerski F, Siebler M, Seitz RJ, Modder U, et al. Diffusion- and perfusion-weighted MRI The DWI/PWI mismatch region in acute stroke. Stroke. 1999; 30:1591–1597. PMID: 10436106.7. Baird AE, Warach S. Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab. 1998; 18:583–609. PMID: 9626183.
Article8. Rohl L, Ostergaard L, Simonsen CZ, Vestergaard-Poulsen P, Andersen G, Sakoh M, et al. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke. 2001; 32:1140–1146. PMID: 11340223.
Article9. Grandin CB, Duprez TP, Smith AM, Mataigne F, Peeters A, Oppenheim C, et al. Usefulness of magnetic resonance-derived quantitative measurements of cerebral blood flow and volume in prediction of infarct growth in hyperacute stroke. Stroke. 2001; 32:1147–1153. PMID: 11340224.
Article10. Grandin CB, Duprez TP, Smith AM, Oppenheim C, Peeters A, Robert AR, et al. Which MR-derived perfusion parameters are the best predictors of infarct growth in hyperacute stroke? Comparative study between relative and quantitative measurements. Radiology. 2002; 223:361–337. PMID: 11997538.
Article11. Neumann-Haefelin T, Wittsack HJ, Fink GR, Wenserski F, Li TQ, Seitz RJ, et al. Diffusion- and perfusion-weighted MRI: influence of severe carotid artery stenosis on the DWI/PWI mismatch in acute stroke. Stroke. 2000; 31:1311–1317. PMID: 10835450.12. Yamada K, Wu O, Gonzalez RG, Bakker D, Ostergaard L, Copen WA, et al. Magnetic resonance perfusion-weighted imaging of acute cerebral infarction: effect of the calculation methods and underlying vasculopathy. Stroke. 2002; 33:87–94. PMID: 11779894.13. Ferrari M, Wilson DA, Hanley DF, Traystman RJ. Effects of graded hypotension on cerebral blood flow, blood volume, and mean transit time in dogs. Am J Physiol. 1992; 262:H1908–H1914. PMID: 1621847.
Article14. Zaharchuk G, Mandeville JB, Bogdanov AA Jr, Weissleder R, Rosen BR, Marota JJ. Cerebrovascular dynamics of autoregulation and hypoperfusion. An MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke. 1999; 30:2197–2204. PMID: 10512929.15. Gibbs JM, Leenders KL, Wise RJ, Jones T. Evaluation of cerebral perfusion reserve in patients with carotid-artery occlusion. Lancet. 1984; 1:182–186. PMID: 6141333.
Article16. Schumann P, Touzani O, Young AR, Morello R, Baron JC, Mackenzie ET. Evaluation of the ratio of cerebral blood flow to cerebral blood volume as an index of local cerebral perfusion pressure. Brain. 1998; 121:1369–1379. PMID: 9679787.17. Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991; 29:231–240. PMID: 2042939.
Article18. Sette G, Baron JC, Mazoyer B, Levasseur M, Pappata S, Crouzel C. Local brain haemodynamics and oxygen metabolism in cerebrovascular disease: positron emission tomography. Brain. 1989; 112:931–951. PMID: 2789086.19. Liu Y, Karonen JO, Vanninen RL, Ostergaard L, Roivainen R, Nuutinen J, et al. Cerebral hemodynamics in human acute ischemic stroke: a study with diffusion- and perfusion-weighted magnetic resonance imaging and SPECT. J Cereb Blood Flow Metab. 2000; 20:910–920. PMID: 10894174.
Article20. Schaefer PW, Ozsunar Y, He J, Hamberg LM, Hunter GJ, Sorensen AG, et al. Assessing tissue viability with MR diffusion and perfusion imaging. AJNR Am J Neuroradiol. 2003; 24:436–443. PMID: 12637294.21. Heiss WD, Podreka I. Wagner HN, Szabo Z, Buchanan JW, editors. Cerebrovascular disease. Principles of nuclear medicine. 1995. Philadelphia, Pa: WB Saunders Co;p. 531–548.22. Desmond PM, Lovell AC, Rawlinson AA, Parsons MW, Barber PA, Yang Q, et al. The value of apparent diffusion coefficient maps in early cerebral ischemia. AJNR Am J Neuroradiol. 2001; 22:1260–1267. PMID: 11498412.23. Oppenheim C, Grandin C, Samson Y, Smith A, Duprez T, Marsault C, et al. Is there an apparent diffusion coefficient threshold in predicting tissue viability in hyperacute stroke? Stroke. 2001; 32:2486–2491. PMID: 11692005.
Article24. Busch E, Kruger K, Allegrini PR, Kerskens CM, Gyngell ML, Hoehn-Berlage M, et al. Reperfusion after thrombolytic therapy of embolic stroke in the rat: magnetic resonance and biochemical imaging. J Cereb Blood Flow Metab. 1998; 18:407–418. PMID: 9538906.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imaging Diagnosis of Occlusive Cerebrovascular Diseases and Cerebral Infarction
- Effect of Gd-DTPA on Diffusion in Canine Brain with Hyperacute Stroke
- Hypointensity on Susceptibility-Weighted Images Prior to Signal Change on Diffusion-Weighted Images in a Hyperacute Ischemic Infarction: a Case Study
- Assessment of Tissue Viability in Hyperacute Infarction with Using the Diffusion- and Perfusion-weighted Images
- Benign Oligemia Despite a Malignant MRI Profile in Acute Ischemic Stroke