J Korean Med Sci.
2009 Dec;24(6):1126-1131. 10.3346/jkms.2009.24.6.1126.
Removal of Alpha-Gal Epitopes from Porcine Aortic Valve and Pericardium using Recombinant Human Alpha Galactosidase A
- Affiliations
-
- 1Department of Cardiothoracic Surgery, Dankook University Hospital, College of Medicine, Dankook University, Cheonan, Korea.
- 2Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. kyj@plaza.snu.ac.kr
- KMID: 1783143
- DOI: http://doi.org/10.3346/jkms.2009.24.6.1126
Abstract
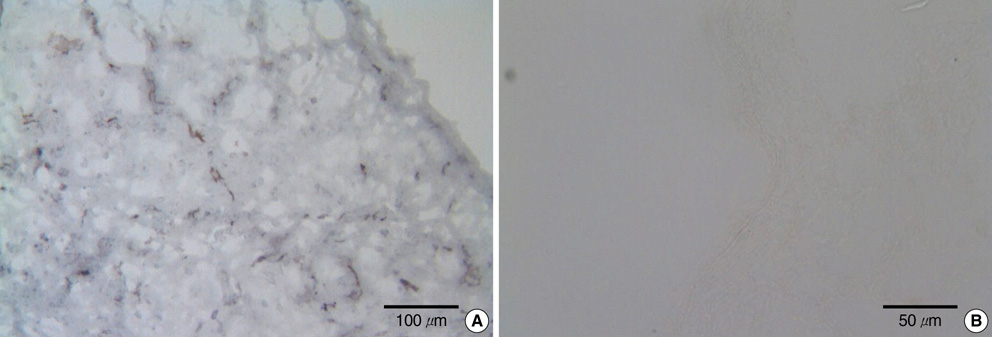
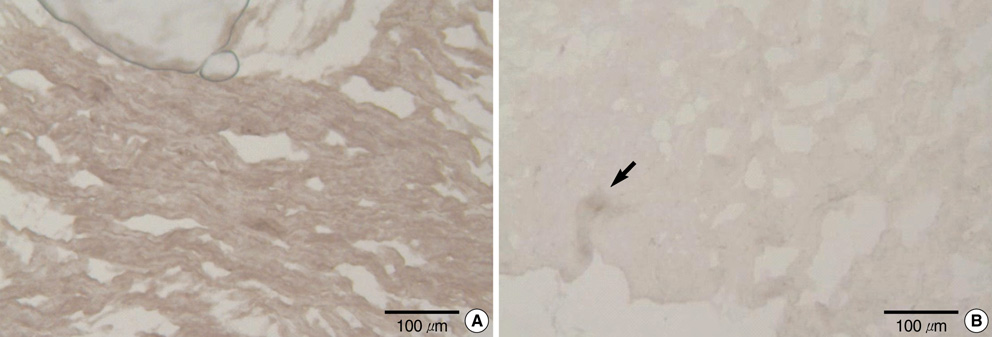
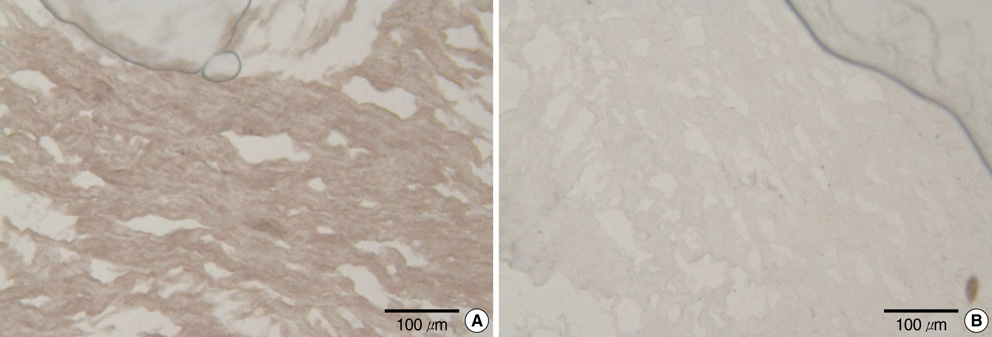
- It has been reported that the immune response due to alpha-Gal epitopes is an important factor in tissue valve failure. The elimination of the interaction between the natural anti-Gal antibodies and alpha-gal epitopes on the xenografts is a prerequisite to the success of xenografts in humans. Previously, we reported that the green coffee bean alpha-galactosidase could remove all alpha-Gal epitopes from cell surface of porcine aortic valve and pericardial tissue, but it has limitations on cost effectiveness. In this study we wanted to know whether the recently produced recombinant human alpha-galactosidase A has the same effective enzymatic activity as green coffee bean alpha-galactosidase in removing alpha-Gal epitopes from the same tissues. After treating fresh porcine aortic valve and pericardial tissue with recombinant alpha-galactosidase A, each sample was stained with Griffonia simplicifolia type I isolectin B4 indirect immunoperoxidase avidin-biotin technique. We then examined whether the alpha-Gal epitopes were reduced or abolished in each consecutive concentration of recombinant alpha-galactosidase A by comparing the degree of the Griffonia simplicifolia isolectin B4 staining. As a result, the recombinant alpha-galactosidase A could remove cell surface alpha-Gals on porcine aortic valve and pericardial tissue as effectively as green coffee bean alpha-galactosidase.
MeSH Terms
-
Adolescent
Animals
Aortic Valve/chemistry/cytology/*immunology
Child
Coffea/enzymology
Epitopes/*immunology
Heart Valve Prosthesis Implantation
Humans
Pericardium/chemistry/cytology/*immunology
Recombinant Proteins/genetics/*immunology
Swine
Transplantation, Heterologous/immunology
alpha-Galactosidase/genetics/*immunology
Figure
Reference
-
1. Jamieson WR, Rosado LJ, Munro AI, Gerein AN, Burr LH, Miyagishima RT, Janusz MT, Tyers GF. Carpentier-Edwards standard porcine bioprosthesis: primary tissue failure (structural valve deterioration) by age groups. Ann Thorac Surg. 1988. 46:155–162.
Article2. Konakci KZ, Bohle B, Blumer R, Hoetzenecker W, Roth G, Moser B, Boltz-Nitulescu G, Gorlitzer M, Klepetko W, Wolner E, Ankersmit HJ. Alpha-Gal on bioprosthesis: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005. 35:17–23.3. Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993. 56:1433–1442.4. Goldstein IJ, Blake DA, Ebisu S, Williams TJ, Murphy LA. Carbohydrate binding studies on the Bandeiraea simplicifolia 1 isolectins. Lectins which are mono-, di-, tri-, and tetravalent for N-acetyl-D-galactosamine. J Biol Chem. 1981. 256:3890–3893.5. Goldstein IJ, Winter HG. The Griffonia simplicifolia 1-B4 isolectin. A probe for alpha-D-galactosyl end groups. Subcell Biochem. 1999. 32:127–141.6. Kirkeby S, Moe D. Binding of Griffonia simplicifolia 1 isolectin B4 (GS1 B4) to α-galactose antigens. Immunol Cell Biol. 2001. 79:121–127.7. LaVecchio JA, Dunne AD, Edge AS. Enzymatic removal of alpha-galactosyl epitopes from porcine endothelial cells diminishes the cytotoxic effect of natural antibodies. Transplantation. 1995. 60:841–847.
Article8. Stone KR, Ayala G, Goldstein J, Hurst R, Walgenbach A, Galili U. Porcine cartilage transplants in the cynomolgus monkey: III Transplantation of α-galactosidase treated porcine cartilage. Transplantation. 1998. 65:1577–1583.9. Luo Y, Wen J, Luo C, Cummings RD, Cooper DK. Pig xenogeneic antigen modification with green coffee bean alpha-galactosidase. Xenotransplantation. 1999. 6:238–248.10. Park S, Kim WH, Kim KH, Lee CH, Choi SY, Lee C, Oh SS, Kim KC, Kim YJ. Removal of alpha-Gal epitopes in aortic valve and pericardium of pig using green coffee bean α-galactosidase. Kor J Thorac Cardiovasc Surg. 2008. 41:12–24.11. Treasure T. Rethink on biological aortic valves for the elderly. Lancet. 1999. 354:964–965.
Article12. The United Kingdom Heart Valve Registry Report 1997. 1999. London: UK Heart valve registry, Imperial College School of Medicine, Hammersmith Hospital.13. David TE, Ivanov J. Is degenerative calcification of the native aortic valve similar to calcification of bioprosthetic heart valves? J Thorac Cardiovasc Surg. 2003. 126:939–941.
Article14. Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of (α)1,3-galactosyltransferase deficient pigs. Science. 2003. 299:411–414.15. Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, Monahan JA, Jobst PM, McCreath KJ, Lamborn AE, Cowell-Lucero JL, Wells KD, Colman A, Polejaeva IA, Ayares DL. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002. 20:251–255.16. Pisani A, Spinelli L, Sabbatini M, Andreucci MV, Procaccini D, Abbaterusso C, Pasquali S, Savoldi S, Comotti C, Cianciaruso B. Enzyme replacement therapy in Fabry disease patients undergoing dialysis: effects on quality of life and organ involvement. Am J Kidney Dis. 2005. 46:120–127.
Article17. Wanner C. Fabry disease model: a rational approach to the management of Fabry disease. Clin Ther. 2007. 29:Suppl A. S2–S5.
Article18. Galili U, Latemple DC, Radic MZ. A sensitive assay for measuring alpha-Gal epitope expression on cells by a monoclonal anti-Gal antibody. Transplantation. 1998. 65:1129–1132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Removal of alpha-Gal Epitopes in Aortic Valve and Pericardium ofPig Using Green Coffee Bean alpha-Galactosidase
- Changes of the Structural and Biomechanical Properties of the Bovine Pericardium after the Removal of alpha-Gal Epitopes by Decellularization and alpha-Galactosidase Treatment
- Histologic Characteristics and Mechanical Properties of Bovine Pericardium Treated with Decellularization and alpha-Galactosidase: A Comparative Study
- The Characteristics of Porcine Cornea as a Xenograft
- Fabry Cardiomyopathy