J Korean Soc Magn Reson Med.
2010 Jun;14(1):31-40. 10.13104/jksmrm.2010.14.1.31.
Ferucarbotran-Enhanced Hepatic MRI at 3T Unit: Quantitative and Qualitative Comparison of Fast Breath-hold Imaging Sequences
- Affiliations
-
- 1Department of Radiology and Institute of Radiological Science, Yonsei University College of Medicine, Gangnam Severance Hospital, Seoul, Korea. yjsrad97@yuhs.ac
- KMID: 1782991
- DOI: http://doi.org/10.13104/jksmrm.2010.14.1.31
Abstract
- PURPOSE
To compare the relative values of various fast breath-hold imaging sequences for superparamagnetic iron-oxide (SPIO)-enhanced hepatic MRI for the assessment of solid focal lesions with a 3T MRI unit.
MATERIALS AND METHODS
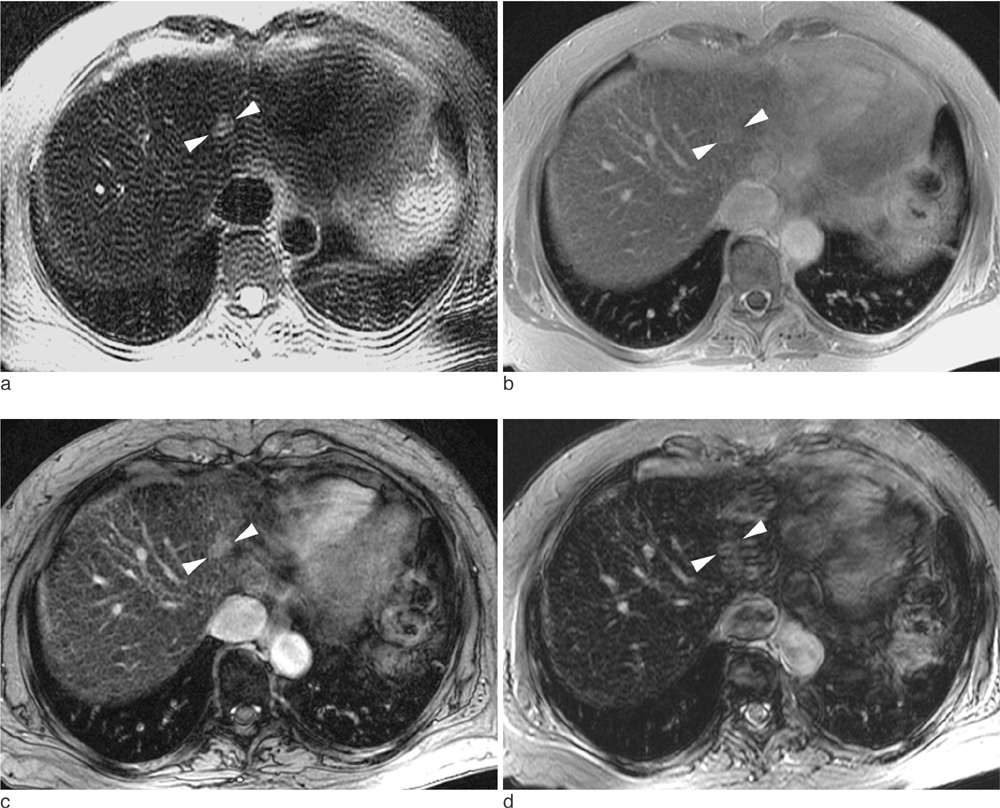
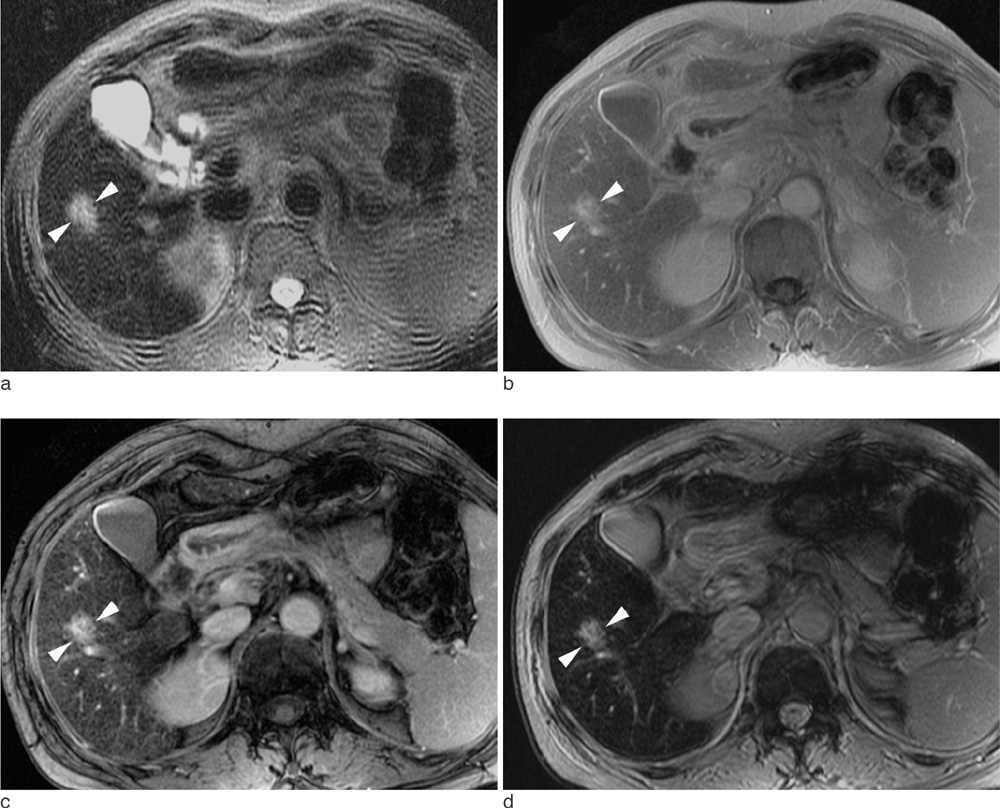
102 consecutive patients with one or more solid malignant hepatic lesions were evaluated by spoiled gradient echo (GRE) sequences with three different echo times (2.4 msec [GRE_2.4], 5.8 msec [GRE_5.8], and 10 msec [GRE_10]) for T2*-weighted imaging in addition to T2-weighted turbo spin echo (TSE) sequence following intravenous SPIO injection. Image qualities of the hepatic contour, vascular landmarks and artifacts were rated by two independent readers using a four-point scale. For quantitative analysis, contrast-to-noise ratio (CNR) was measured in 170 solid focal lesions larger than 1 cm (107 hepatocellular carcinomas, nine cholangiocarcinomas and 54 metastases).
RESULTS
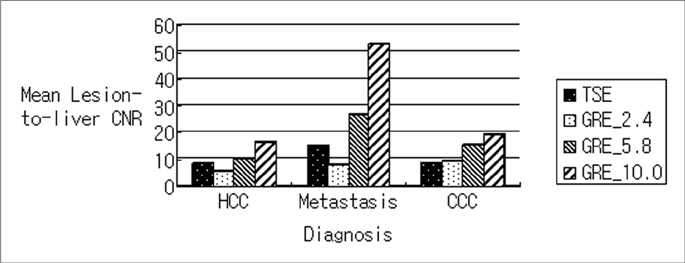
GRE_5.8 showed the highest mean points for hepatic contour, vascular anatomy and imaging artifact presence among all of the subjected sequences (p<0.001) and was comparable (p=0.414) with GRE_10 with regard to lesion conspicuity. The mean CNRs were significantly higher (p<0.001) in the following order: GRE_10 (24.4+/-14.5), GRE_5.8 (14.8+/-9.4), TSE (9.7+/-6.3), and GRE_2.4 (7.9+/-6.4). The mean CNRs of CCCs and metastases were higher than those of HCCs for all imaging sequences (p<0.05).
CONCLUSION
Regarding overall performances, GRE using a moderate echo time of 5.8 msec can provide the most reliable data among the various fast breath-hold SPIO-enhanced hepatic MRI sequences at 3T unit despite the lower CNR of GRE_5.8 compared to that of GRE_10.
MeSH Terms
Figure
Reference
-
1. Van Beers BE, Gallez B, Pringot J. Contrast-enhanced MR iaging of the liver. Radiology. 1997. 203:297–306.2. Reimer P, Tombach B. Hepatic MRI with SPIO: detection and characterization of focal liver lesions. Eur Radiol. 1998. 8:1198–1204.3. von Falkenhausen M, Meyer C, Lutterbey G, et al. Intraindividual comparison of image contrast in SPIO-enhanced liver MRI at 1.5T and 3.0T. Eur Radiol. 2007. 17:1256–1261.4. Chang JM, Lee JM, Lee MW, et al. Superparamagnetic iron oxide-enhanced liver magnetic resonance imaging-comparison of 1.5T and 3.0T imaging for detection of focal malignant liver lesions. Invest Radiol. 2006. 41:168–174.5. Ramalho M, Altun E, Here′dia V, Zapparoli M, Semelka R. Liver MR imaging: 1.5T versus 3T. Magn Reson Imaging Clin N Am. 2007. 15:321–334.6. von Falkenhausen MM, Lutterbey G, Morakkabati-Spitz N, et al. High-field-strength MR imaging of the liver at 3.0T: Intraindividual comparative study with MR imaging at 1.5T. Radiology. 2006. 241:156–166.7. Kim T, Murakami T, Hori M, Onishi H, Tomoda K, Nakamura H. Effect of superparamagnetic iron oxide on tumor-to-liver contrast at T2*-weighted gradient-echo MRI: comparison between 3s. J Magn Reson Imaging. 2009. 29:595–600.8. Nakada T. Clinical Experience on 3.0T systems in Niigata, 1996 to 2002. Invest Radiol. 2003. 38:377–338.9. Ward J, Guthrie JA, Wilson D, et al. Colorectal hepatic metastases: detection with SPIO-enhanced breath-hold MR imaging-comparison of optimized sequences. Radiology. 2003. 228:709–718.10. Kim MJ, Kim JH, Choi JY, et al. Optimal TE for SPIOenhanced gradient-recalled echo MRI for the detection of focal hepatic lesions. AJR Am J Roentgenol. 2006. 187:W255–W266.11. de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR Imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0T : preliminary results. Radiology. 2004. 230:652–665.12. Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys. 1984. 11:425–448.13. Zapparoli M, Semelka RC, Altun E, et al. 3.0-T MRI evaluation of patients with chronic liver diseases : initial observations. Magn Reson Imaging. 2008. 26:650–660.14. Hahn PF, Stark DD, Weissleder R, Elizondo G, Saini S, Ferrucci JT. Clinical application of superparamagnetic iron oxide to MR imaging of tissue perfusion in vascular liver tumors. Radiology. 1990. 174:361–366.15. Schneider G, Reimer P, Mabmann A, Kirchin MA, Morana G, Grazioli G. Contrast agents in abdominal imaging-current and future directions. Top Magn Reson Imaging. 2005. 16:107–124.16. Bellin MF, Zaim S, Auberton E, et al. Liver metastases: safety and Efficacy of detection with superparamagnetic iron oxide in MR imaging. Radiology. 1994. 193:657–663.17. Matsuo M, Kanematsu M, Itoh K, et al. Detection of malignant hepatic tumors with ferumoxides-enhanced MRI: Comparison of five gradient-recalled echo sequences with different TEs. AJR Am J Roentgenol. 2004. 182:235–242.18. Yoshikawa T, Mitchell DG, Hirota S, et al. Gradient- and spin-echo T2-weighted imaging for SPIO-enhanced detection and characterization of focal liver lesions. J Magn Reson Imaging. 2006. 23:712–719.19. Ward J, Chen F, Guthrie JA, et al. Hepatic lesion detection after superparamagnetic iron oxide enhancement: comparison of five T2-weighted sequences at 1.0 T by using alternativefree response receiver operating characteristic analysis. Radiology. 2000. 214:159–166.20. Kurokawa H, Togami I, Tsunoda M, Hiraki Y. Experimental study of fast and ultrafast T2-weighted imaging sequences using AMI-25 superparamagnetic iron oxide (SPIO). Acta Med Okayama. 2001. 55:41–50.21. Kumano S, Murakami T, Kim T, et al. Using superparamagnetic iron oxide-enhanced MRI to differentiate metastatic hepatic tumors and nonsolid benign lesions. AJR Am J Roentgenol. 2003. 181:1335–1339.22. Tanimoto A, Yuasa Y, Shinmoto H, et al. Superparamagnetic iron oxide-mediated hepatic signal intensity change in patients with and without cirrhosis: pulse sequence effects and Kupffer cell function. Radiology. 2002. 222:661–666.23. Kanematsu M, Itoh K, Matsuo M, et al. Malignant hepatic tumor detection with ferumoxides-enhanced MR imaging with a 1.5-T system: comparison of four imaging pulse sequences. J Magn Reson Imaging. 2001. 13:249–257.24. Elizondo G, Weissleder R, Stark DD, et al. Hepatic cirrhosis and hepatitis: MR imaging enhanced with superparamagnetic iron oxide. Radiology. 1990. 174:797–801.25. Zech CJ, Herrmann KA, Dietrich O, Horger W, Reiser MF, Schoenberg SO. Black-blood diffusion-weighted EPI acquisition of the liver with paraller imaging. Comparison with a standard T2-weighted sequence for detection of focal liver lesions. Invest Radiol. 2008. 43:261–266.26. Kuwatsuru R, Brasch RC, Muhler A, et al. Definition of liver tumors in the presence of diffuse liver disease: comparison of findings at MR imaging with positive and negative contrast agents. Radiology. 1997. 202:131–138.27. Tanimoto A, Yuasa Y, Shinmoto H, et al. Superparamagnetic iron oxide-mediated hepatic signal intensity change in patients with and without cirrhosis: pulse sequence and Kupffer cell function. Radiology. 2002. 222:661–666.28. Tanimoto A, Oshio K, Suematsu M, Pouliquen D, Stark DD. Relaxation effects of clustered particles. J Magn Reson Imaging. 2001. 14:72–77.29. Tang Y, Yamashita Y, Arakawa A, et al. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium and ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 1999. 172:1547–1554.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Non-Breath-Hold T2-weighted Turbo Spin-Echo and Three Breath-Hold T2-weighted MR Images for Detection of Focal Hepatic Lesion
- SPIO-enhanced MR Imaging for HCC Detection in Cirrhotic Patient: Comparison of Various Techniques for Optimal Sequence Selection
- Optimal MR Pulse Sequences for Hepatic Hemangiomas: Comparison of T2-Weighted Turbo-Spin-Echo, T2-Weighted Breath-hold Turbo-Spin-Echo, and T1-Weighted FLASH Dynamic Imaging
- Breath-Hold MR Imaging of Focal Hepatic Lesions: Clinical Usefulness of Breath-Hold TSE T2WI Combined by FastLow-Angle Shot (FLASH) MR Imaging
- The Value of True FISP Sequence as a Fast T2-Weighted MR Imaging of Liver: Comparison with Breath-hold Turbo Spin Echo and HASTE Sequence