Yonsei Med J.
2009 Feb;50(1):22-30. 10.3349/ymj.2009.50.1.22.
Chitin, Chitinases and Chitinase-like Proteins in Allergic Inflammation and Tissue Remodeling
- Affiliations
-
- 1Section of Pulmonary and Critical Care Medicine, Yale University School of Medicine, Cedar Street, New Haven, CT, USA. chungeun.lee@yale.edu
- KMID: 1782963
- DOI: http://doi.org/10.3349/ymj.2009.50.1.22
Abstract
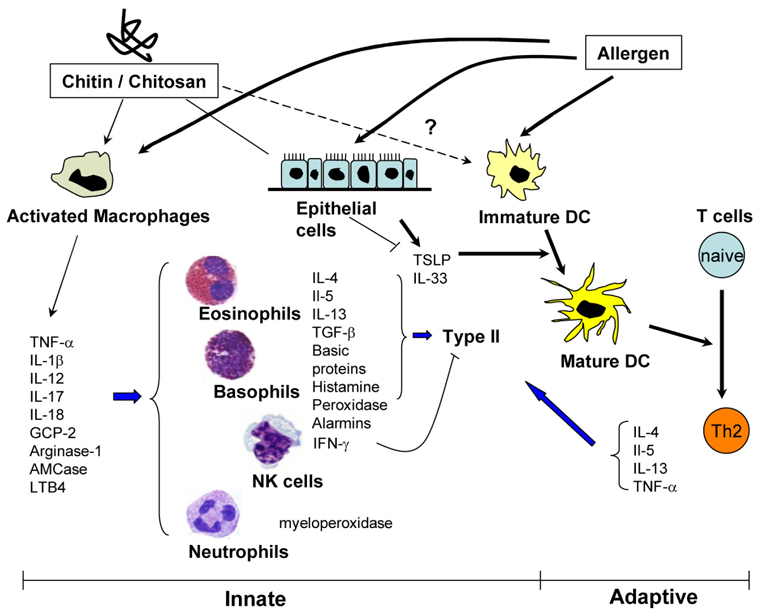
- Chitin, the second most abundant polysaccharide in nature after cellulose, consist exoskeleton of lower organisms such as fungi, crustaceans and insects except mammals. Recently, several studies evaluated immunologic effects of chitin in vivo and in vitro and revealed new aspects of chitin regulation of innate and adaptive immune responses. It has been shown that exogenous chitin activates macrophages and other innate immune cells and also modulates adaptive type 2 allergic inflammation. These studies further demonstrate that chitin stimulate macrophages by interacting with different cell surface receptors such as macrophage mannose receptor, toll-like receptor 2 (TLR-2), C-type lectin receptor Dectin-1, and leukotriene B4 recepptor (BLT1). On the other hand, a number of chitinase or chitinase-like proteins (C/CLP) are ubiquitously expressed in the airways and intestinal tracts from insects to mammals. In general, these chitinase family proteins confer protective functions to the host against exogenous chitin-containing pathogens. However, substantial body of recent studies also set light on new roles of C/CLP in the development and progression of allergic inflammation and tissue remodeling. In this review, recent findings on the role of chitin and C/CLP in allergic inflammation and tissue remodeling will be highlighted and controversial and unsolved issues in this field of studies will be discussed.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Endotoxin Is Not Essential for the Development of Cockroach Induced Allergic Airway Inflammation
Yoo Seob Shin, Jung-Ho Sohn, Joo-Young Kim, Jae Hyun Lee, Sang-Heon Cho, Soo-Jong Hong, Joo-Shil Lee, Chein-Soo Hong, Jung-Won Park
Yonsei Med J. 2012;53(3):593-602. doi: 10.3349/ymj.2012.53.3.593.Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes
Bradford S. Powell, Alexander K. Andrianov, Peter C. Fusco
Clin Exp Vaccine Res. 2015;4(1):23-45. doi: 10.7774/cevr.2015.4.1.23.
Reference
-
1. Araujo AC, Souto-Padrón T, de Souza W. Cytochemical localization of carbohydrate residues in microfilariae of Wuchereria bancrofti and Brugia malayi. J Histochem Cytochem. 1993. 41:571–578.
Article2. Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001. 276:6770–6778.
Article3. Boot RG, Renkema GH, Verhoek M, Strijland A, Bliek J, de Meulemeester TM, et al. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem. 1998. 273:25680–25685.4. Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994. 48:471–497.
Article5. Elias JA, Homer RJ, Hamid Q, Lee CG. Chitinases and chitinase-like proteins in T(H)2 inflammation and asthma. J Allergy Clin Immunol. 2005. 116:497–500.
Article6. Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Mol Biochem Parasitol. 1985. 17:93–104.
Article7. Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J Cell Sci. 1976. 21:73–82.
Article8. Shahabuddin M, Kaslow DC. Plasmodium: parasite chitinase and its role in malaria transmission. Exp Parasitol. 1994. 79:85–88.
Article9. Burton OT, Zaccone P. The potential role of chitin in allergic reactions. Trends Immunol. 2007. 28:419–422.
Article10. Muzzarelli RA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol Life Sci. 1997. 53:131–140.
Article11. Myles O, Wortmann GW, Cummings JF, Barthel RV, Patel S, Crum-Cianflone NF, et al. Visceral leishmaniasis: clinical observations in 4 US army soldiers deployed to Afghanistan or Iraq, 2002-2004. Arch Intern Med. 2007. 167:1899–1901.
Article12. Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, Chen S, et al. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L502–L511.
Article13. Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006. 130:398–411.
Article14. Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004. 304:1678–1682.
Article15. Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inflammatory conditions. Keio J Med. 2007. 56:21–27.
Article16. Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006. 53:172–209.17. Bierbaum S, Nickel R, Koch A, Lau S, Deichmann KA, Wahn U, et al. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am J Respir Crit Care Med. 2005. 172:1505–1509.
Article18. Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007. 357:2016–2027.
Article19. Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008. 358:1682–1691.
Article20. Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. Immunological activity of chitin and its derivatives. Vaccine. 1984. 2:93–99.
Article21. Nishimura S, Nishi N, Tokura S, Nishimura K, Azuma I. Bioactive chitin derivatives. Activation of mouse-peritoneal macrophages by O-(carboxymethyl)chitins. Carbohydr Res. 1986. 146:251–258.
Article22. Suzuki K, Okawa Y, Hashimoto K, Suzuki S, Suzuki M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol Immunol. 1984. 28:903–912.
Article23. Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974. 59:1317–1325.
Article24. Azuma I, Sugimura K, Taniyama T, Yamawaki M, Yamamura Y. Adjuvant activity of mycobacterial fractions: adjuvant activity of synthetic N-acetylmuramyl-dipeptide and the related compounds. Infect Immun. 1976. 14:18–27.
Article25. Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol. 1997. 159:2462–2467.26. Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect Immun. 1997. 65:1734–1741.
Article27. Bourbouze R, Raffi F, Dameron G, Hali-Miraftab H, Loko F, Vilde JL. N-acetyl-beta-D-glucosaminidase (NAG) isoenzymes release from human monocyte-derived macrophages in response to zymosan and human recombinant interferon-gamma. Clin Chim Acta. 1991. 199:185–194.28. Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007. 447:92–96.
Article29. Zhang Y, Matsuo H, Morita E. Cross-reactivity among shrimp, crab and scallops in a patient with a seafood allergy. J Dermatol. 2006. 33:174–177.
Article30. Desjardins A, Malo JL, L'Archevêque J, Cartier A, McCants M, Lehrer SB. Occupational IgE-mediated sensitization and asthma caused by clam and shrimp. J Allergy Clin Immunol. 1995. 96:608–617.31. Shibata Y, Foster LA, Bradfield JF, Myrvik QN. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000. 164:1314–1321.32. Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger WJ. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J Immunol. 1996. 157:4173–4180.33. Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995. 182:1527–1536.34. Shibata Y, Honda I, Justice JP, Van Scott MR, Nakamura RM, Myrvik QN. Th1 adjuvant N-acetyl-D-glucosamine polymer up-regulates Th1 immunity but down-regulates Th2 immunity against a mycobacterial protein (MPB-59) in interleukin-10-knockout and wild-type mice. Infect Immun. 2001. 69:6123–6130.35. Hamajima K, Kojima Y, Matsui K, Toda Y, Jounai N, Ozaki T, et al. Chitin Micro-Particles (CMP): a useful adjuvant for inducing viral specific immunity when delivered intranasally with an HIV-DNA vaccine. Viral Immunol. 2003. 16:541–547.
Article36. Strong P, Clark H, Reid K. Intranasal application of chitin microparticles down-regulates symptoms of allergic hypersensitivity to Dermatophagoides pteronyssinus and Aspergillus fumigatus in murine models of allergy. Clin Exp Allergy. 2002. 32:1794–1800.37. Ozdemir C, Yazi D, Aydogan M, Akkoc T, Bahceciler NN, Strong P, et al. Treatment with chitin microparticles is protective against lung histopathology in a murine asthma model. Clin Exp Allergy. 2006. 36:960–968.
Article38. Chen CL, Wang YM, Liu CF, Wang JY. The effect of water-soluble chitosan on macrophage activation and the attenuation of mite allergen-induced airway inflammation. Biomaterials. 2008. 29:2173–2182.
Article39. MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008. 205:13–17.
Article40. Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007. 25:193–219.
Article41. Shibaki A, Katz SI. Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund's adjuvant. Exp Dermatol. 2002. 11:126–134.
Article42. Lee CG, Hartl D, Matsuura H, Dunlop FM, Scotney PD, Fabri LJ, et al. Endogenous IL-11 signaling is essential in Th2- and IL-13-induced inflammation and mucus production. Am J Respir Cell Mol Biol. 2008. 39:739–746.
Article43. Weber E, Hunter S, Stedman K, Dreitz S, Olivry T, Hillier A, et al. Identification, characterization, and cloning of a complementary DNA encoding a 60-kd house dust mite allergen (Der f 18) for human beings and dogs. J Allergy Clin Immunol. 2003. 112:79–86.
Article44. Song HM, Jang AS, Ahn MH, Takizawa H, Lee SH, Kwon JH, et al. Ym1 and Ym2 expression in a mouse model exposed to diesel exhaust particles. Environ Toxicol. 2008. 23:110–116.
Article45. Donnelly LE, Barnes PJ. Acidic mammalian chitinase--a potential target for asthma therapy. Trends Pharmacol Sci. 2004. 25:509–511.46. Musumeci M, Bellin M, Maltese A, Aragona P, Bucolo C, Musumeci S. Chitinase levels in the tears of subjects with ocular allergies. Cornea. 2008. 27:168–173.
Article47. Zhao J, Zhu H, Wong CH, Leung KY, Wong WS. Increased lungkine and chitinase levels in allergic airway inflammation: a proteomics approach. Proteomics. 2005. 5:2799–2807.
Article48. Seibold MA, Donnelly S, Solon M, Innes A, Woodruff PG, Boot RG, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. J Allergy Clin Immunol. 2008. 122:944–950. e3.
Article49. Webb DC, McKenzie AN, Foster PS. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J Biol Chem. 2001. 276:41969–41976.
Article50. Bargagli E, Margollicci M, Nikiforakis N, Luddi A, Perrone A, Grosso S, et al. Chitotriosidase activity in the serum of patients with sarcoidosis and pulmonary tuberculosis. Respiration. 2007. 74:548–552.
Article51. Bargagli E, Margollicci M, Luddi A, Nikiforakis N, Perari MG, Grosso S, et al. Chitotriosidase activity in patients with interstitial lung diseases. Respir Med. 2007. 101:2176–2181.
Article52. Malaguarnera L, Rosa MD, Zambito AM, dell'Ombra N, Marco RD, Malaguarnera M. Potential role of chitotriosidase gene in nonalcoholic fatty liver disease evolution. Am J Gastroenterol. 2006. 101:2060–2069.
Article53. Malaguarnera L, Di Rosa M, Zambito AM, dell'Ombra N, Nicoletti F, Malaguarnera M. Chitotriosidase gene expression in Kupffer cells from patients with non-alcoholic fatty liver disease. Gut. 2006. 55:1313–1320.
Article54. Johansen JS, Christoffersen P, Møller S, Price PA, Henriksen JH, Garbarsch C, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000. 32:911–920.
Article55. Johansen JS, Møller S, Price PA, Bendtsen F, Junge J, Garbarsch C, et al. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand J Gastroenterol. 1997. 32:582–590.
Article56. Zheng M, Cai WM, Zhao JK, Zhu SM, Liu RH. Determination of serum levels of YKL-40 and hyaluronic acid in patients with hepatic fibrosis due to schistosomiasis japonica and appraisal of their clinical value. Acta Trop. 2005. 96:148–152.
Article57. Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF Jr, Cheever AW, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006. 116:2044–2055.
Article58. Migliaccio CT, Buford MC, Jessop F, Holian A. The IL-4Ralpha pathway in macrophages and its potential role in silica-induced pulmonary fibrosis. J Leukoc Biol. 2008. 83:630–639.
Article59. Mora AL, Torres-González E, Rojas M, Corredor C, Ritzenthaler J, Xu J, et al. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol. 2006. 35:466–473.
Article60. Iida J, Une T, Ishihara C, Nishimura K, Tokura S, Mizukoshi N, et al. Stimulation of non-specific host resistance against Sendai virus and Escherichia coli infections by chitin derivatives in mice. Vaccine. 1987. 5:270–274.
Article61. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008. 13:453–461.
Article62. Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008. 181:4279–4286.63. Andersson LI, Hellman P, Eriksson H. Receptor-mediated endocytosis of particles by peripheral dendritic cells. Hum Immunol. 2008. 69:625–633.
Article64. van Vliet SJ, García-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol Cell Biol. 2008. 86:580–587.
Article65. Andersen OA, Dixon MJ, Eggleston IM, van Aalten DM. Natural product family 18 chitinase inhibitors. Nat Prod Rep. 2005. 22:563–579.
Article66. Houston DR, Recklies AD, Krupa JC, van Aalten DM. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes. J Biol Chem. 2003. 278:30206–30212.
Article67. Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003. 278:37753–37760.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chitinase, Chitinase-like Protein and Allergic Inflammation
- Does caffeine have a double-edged sword role in inflammation and carcinogenesis in the colon?
- The Usefulness as a Specific Marker of Blood and Nasal Lavage Fluid YKL-40 in Allergic Rhinitis Patients
- Role of breast regression protein-39/YKL-40 in asthma and allergic responses
- Trichoderma asperellum Chi42 Genes Encode Chitinase