J Korean Med Sci.
2011 Sep;26(9):1231-1237. 10.3346/jkms.2011.26.9.1231.
Clinical and Immunological Responses in Ocular Demodecosis
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Chung-Ang University Hospital, Seoul, Korea. jck50ey@kornet.net
- KMID: 1779400
- DOI: http://doi.org/10.3346/jkms.2011.26.9.1231
Abstract
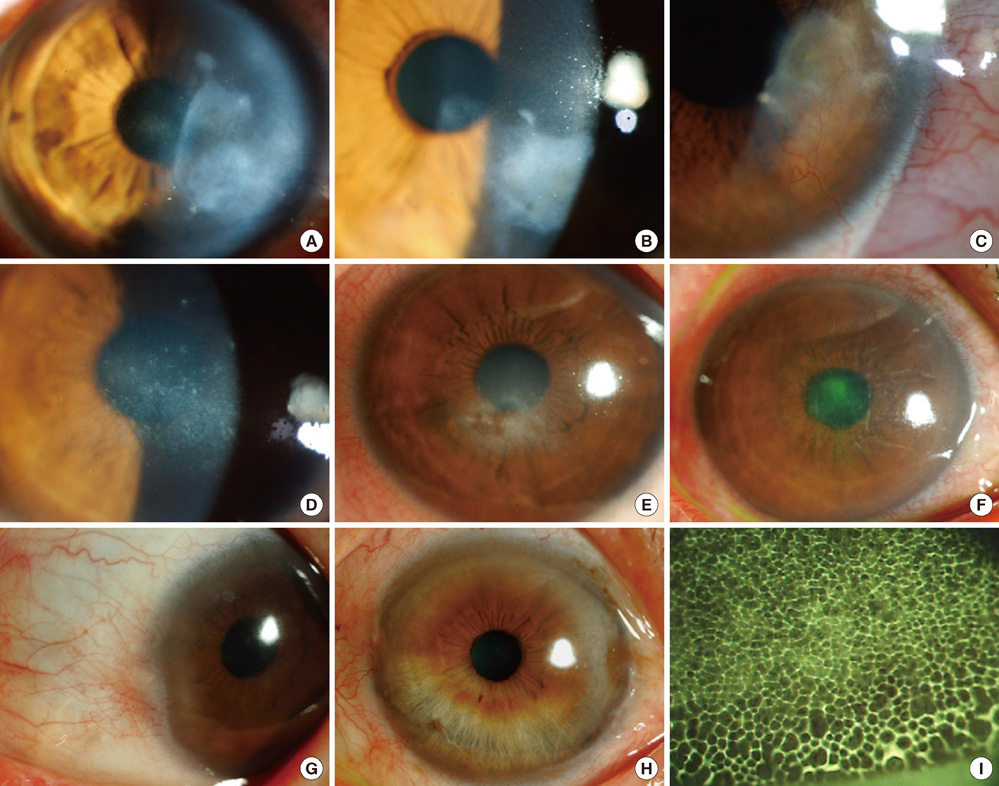
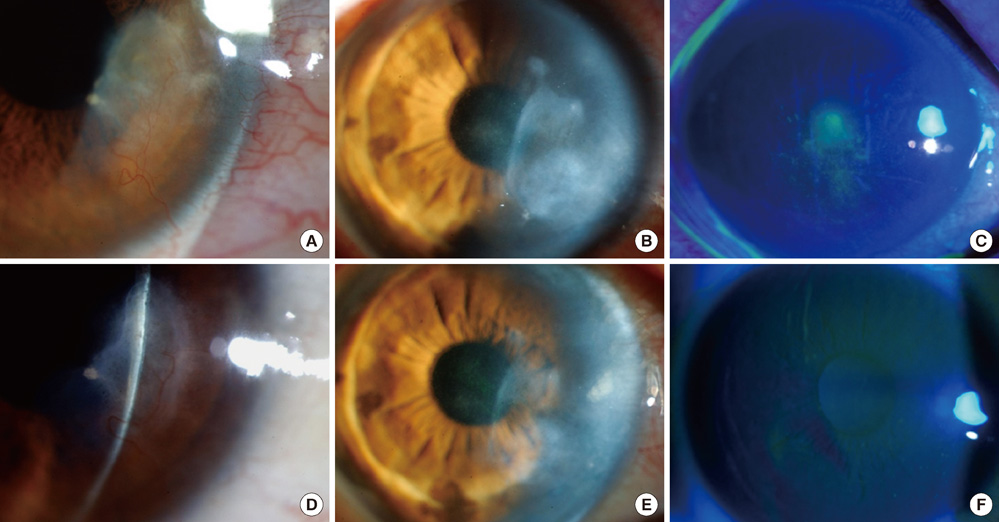
- The purpose of this study was to investigate clinical and immunological responses to Demodex on the ocular surface. Thirteen eyes in 10 patients with Demodex blepharitis and chronic ocular surface disorders were included in this study and treated by lid scrubbing with tea tree oil for the eradication of Demodex. We evaluated ocular surface manifestations and Demodex counts, and analyzed IL-1beta, IL-5, IL-7, IL-12, IL-13, IL-17, granulocyte colony-stimulating factor, and macrophage inflammatory protein-1beta in tear samples before and after the treatment. All patients exhibited ocular surface manifestations including corneal nodular opacity, peripheral corneal vascularization, refractory corneal erosion and infiltration, or chronic conjunctival inflammatory signs before treatment. After treatment, Demodex was nearly eradicated, tear concentrations of IL-1beta and IL-17 were significantly reduced and substantial clinical improvement was observed in all patients. In conclusion, we believe that Demodex plays an aggravating role in inflammatory ocular surface disorders.
Keyword
MeSH Terms
-
Acari/drug effects/physiology
Adolescent
Adult
Aged
Animals
Blepharitis/drug therapy/*immunology/parasitology
Chemokine CCL4/analysis
Female
Granulocyte Colony-Stimulating Factor/analysis
Humans
Interleukin-12/analysis
Interleukin-13/analysis
Interleukin-17/analysis
Interleukin-1beta/analysis
Interleukin-5/analysis
Interleukin-7/analysis
Male
Middle Aged
Tea Tree Oil/therapeutic use
Tears/metabolism
Figure
Cited by 1 articles
-
A Case of Corneal Opacity Improved by Treatment of Demodex Blepharitis
Jung Huh, Kyoung Woo Kim, Jae Chan Kim
J Korean Ophthalmol Soc. 2014;55(10):1558-1561. doi: 10.3341/jkos.2014.55.10.1558.
Reference
-
1. Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002. 82:3–6.2. English FP, Nutting WB. Demodicosis of ophthalmic concern. Am J Ophthalmol. 1981. 91:362–372.3. Coston TO. Demodex folliculorum blepharitis. Trans Am Ophthalmol Soc. 1967. 65:361–392.4. Kheirkhah A, Casas V, Li W, Raju VK, Tseng SC. Corneal manifestations of ocular demodex infestation. Am J Ophthalmol. 2007. 143:743–749.5. Rodríguez AE, Ferrer C, Alió JL. Chronic blepharitis and Demodex. Arch Soc Esp Oftalmol. 2005. 80:635–642.6. Kheirkhah A, Blanco G, Casas V, Tseng SC. Fluorescein dye improves microscopic evaluation and counting of demodex in blepharitis with cylindrical dandruff. Cornea. 2007. 26:697–700.7. Gao YY, Di Pascuale MA, Li W, Liu DT, Baradaran-Rafii A, Elizondo A, Kawakita T, Raju VK, Tseng SC. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005. 46:3089–3094.8. Gao YY, Di Pascuale MA, Li W, Baradaran-Rafii A, Elizondo A, Kuo CL, Raju VK, Tseng SC. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005. 89:1468–1473.9. Gao YY, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea. 2007. 26:136–143.10. Jones DT, Monroy D, Pflugfelder SC. A novel method of tear collection: comparison of glass capillary micropipettes with porous polyester rods. Cornea. 1997. 16:450–458.11. Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM, Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O'Brien TP, Pflugfelder SC, Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006. 25:900–907.12. Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991. 5:2145–2154.13. Strissel KJ, Girard MT, West-Mays JA, Rinehart WB, Cook JR, Brinckerhoff CE, Fini ME. Role of serum amyloid A as an intermediate in the IL-1 and PMA-stimulated signaling pathways regulating expression of rabbit fibroblast collagenase. Exp Cell Res. 1997. 237:275–287.14. Strissel KJ, Rinehart WB, Fini ME. Regulation of paracrine cytokine balance controlling collagenase synthesis by corneal cells. Invest Ophthalmol Vis Sci. 1997. 38:546–552.15. Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999. 19:201–211.16. McGeachy MJ, Anderton SM. Cytokines in the induction and resolution of experimental autoimmune encephalomyelitis. Cytokine. 2005. 32:81–84.17. Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996. 183:2593–2603.18. Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998. 160:3513–3521.19. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005. 6:1123–1132.20. Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007. 19:281–286.21. Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guérin infection. J Immunol. 2007. 178:3786–3796.22. Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002. 17:375–387.23. Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004. 279:2559–2567.24. Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999. 162:494–502.25. Chabaud M, Lubberts E, Joosten L, van Den Berg W, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001. 3:168–177.26. Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999. 162:577–584.27. Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998. 111:645–649.28. Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001. 108:430–438.29. Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001. 167:4137–4140.30. Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003. 101:2620–2627.