J Korean Med Sci.
2007 Jun;22(3):400-404. 10.3346/jkms.2007.22.3.400.
Outpatient-basis Chemotherapy of Oxaliplatin, 5-fluorouracil, and Leucovorin as First-line Treatment for Patients with Metastatic or Recurrent Colorectal Cancer
- Affiliations
-
- 1Department of Oncology/Hematology, Kyungpook National University Hospital, Kyungpook National University School of Medicine, 50 Samduck 2-ga, Jung-gu, Daegu, Korea. jkk21c@knu.ac.kr
- 2Department of General Surgery, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
- 3Department of Oncology/Hematology, Dongguk University Medical Center, Gyeongju, Korea.
- KMID: 1778344
- DOI: http://doi.org/10.3346/jkms.2007.22.3.400
Abstract
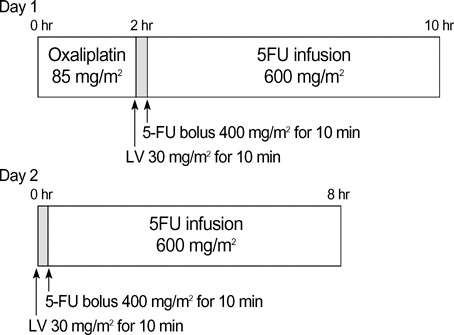
- The objectives of the present study were to evaluate the efficacy and safety of an outpatient-basis chemotherapy of oxaliplatin, 5-fluorouracil, and leucovorin as the first-line treatment for patients with advanced colorectal cancer. Forty-three histologically confirmed patients with metastatic or recurrent colorectal cancer were enrolled. The chemotherapy consisted of oxaliplatin 85 mg/m2 as a 2-hr infusion on day 1, plus leucovorin 30 mg/m2 over 10 min, followed by bolus 5-fluorouracil 400 mg/m2 and an 8-hr infusion of 5-fluorouracil 600 mg/m2 on days 1 and 2 (modified FOLFOX4), all of which were administered on an outpatient basis every 2 weeks. The median age was 58 yr (range 33-72 yr), and 25 (58.1%) patients had metastatic diseases. Eventually, 39 patients were assessable for efficacy and all assessable for toxicity. Four (9.3%) complete responses and 11 (25.6%) partial responses were confirmed, giving an overall response rate of 34.9% (95% CI; 20.0-49.7%). The median time to progression and median overall survival for all patients was 6.1 months and 17.4 months, respectively. Grade 3/4 neutropenia occurred in 2 patients (4.7%) and febrile neutropenia was observed in 1 patient (2.3%). Modified FOLFOX4, an outpatient-basis regimen, was found to be well-tolerated and effective as the firstline chemotherapy in patients with advanced colorectal cancer.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Antineoplastic Combined Chemotherapy Protocols/*therapeutic use
Colorectal Neoplasms/*drug therapy/pathology
Female
Fluorouracil/*administration & dosage
Humans
Leucovorin/*administration & dosage
Male
Middle Aged
Neoplasm Metastasis
Organoplatinum Compounds/*administration & dosage
Outpatients
Recurrence
Figure
Reference
-
1. American Cancer Society. Cancer Facts and Figures-2000. 2000. Atlanta, GA: American Cancer Society.2. de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, Morvan F, Louvet C, Guillot T, Francois E, Bedenne L. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997. 15:808–815.
Article3. Andre T, Colin P, Louvet C, Gamelin E, Bouche O, Achille E, Colbert N, Boaziz C, Piedbois P, Tubiana-Mathieu N, Boutan-Laroze A, Flesch M, Buyse M, de Gramont A. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol. 2003. 21:2896–2903.4. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000. 18:2938–2947.
Article5. Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Levi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000. 18:136–147.
Article6. Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG, Le Bail N, Haller DG. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003. 21:2059–2069.
Article7. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004. 22:23–30.
Article8. Nancy K, Carlos AG, Jayne G, Howard H, Peter K, Al B, Debra SB, Jonathan P, Michael W, Grace S, Doreen H, Brent B, Sunil G. Randomized multicenter phase II trial of bolus plus infusional fluorouracil/ leucovorin compared with fluorouracil/leucovorin plus oxaliplatin as third-line treatment of patients with advanced colorectal cancer. J Clin Oncol. 2004. 22:4753–4761.9. Yves B, Erick G, Bruno C, Sylvie N, Jean-Yves P, Jean-Luc R, Jocelyne P, Olivier R, Claude K, Caroline G, Mohamed B, Dominique M, Mireille M. Randomized multicenter phase II study comparing a combination of fluorouracil and folinic acid and alternating irinotecan and oxaliplatin with oxaliplatin and irinotecan in fluorouracil-pretreated metastatic colorectal cancer patients. J Clin Oncol. 2001. 19:4195–4201.10. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.11. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989. 10:1–10.
Article12. Ramanathan RK, Clark JW, Kemeny NE, Lenz HJ, Gococo KO, Haller DG, Mitchell EP, Kardinal CG. Safety and toxicity analysis of oxaliplatin combined with fluorouracil or as a single agent in patients with previously treated advanced colorectal cancer. J Clin Oncol. 2003. 21:2904–2911.
Article13. Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999. 17:3560–3568.14. Kwon HC, Kim KT, Lee SA, Park JS, Kim SH, Kim JS, Kim HJ. Oxaliplatin with biweekly, low dose leucovorin and bolus and continuous Infusion 5-fluorouracil (Modified FOLFOX 4) as First-line therapy for patients with metastatic colorectal cancer. Cancer Research Treatment. 2004. 36:115–120.
Article15. Shin YM, Han HS, Lim SW, Kim BC, Cheoi KS, Eum YO, Kim ST, Lee KH. Combination chemotherapy of oxaliplatin, 5-fluorouracil and low dose leucovorin in patients with advanced colorectal cancer. Cancer Res Treat. 2005. 37:284–289.
Article16. Lee JH, Kim TW, Lee KH, Kang YK, Lee JS, Kim SH, Kim HC, Yu CS, Kim JC, Kim WK. Combination of oxaliplatin, fluorouracil, and leucovorin in the treatment of fluoropyrimidine-pretreated patients with metastatic colorectal cancer. J Korean Med Sci. 2001. 16:69–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Leucovorin-induced Hypersensitivity Reaction in a Patient with Metastatic Colorectal Cancer Treated with Cetuximab Plus FOLFOX Chemotherapy: A Case Report
- Comparison between Responder and Non- responder of Oxaliplatin Chemotherapy for Metastatic Colorectal Cancer
- 5-fluorouracil and low dose leucovorin combination chemotherapy for metastatic or recurrent colorectal cancer
- Tumor Lysis Syndrome in a Patient with Metastatic Colon Cancer after Treatment with 5-Fluorouracil/Leucovorin and Oxaliplatin: Case Report and Literature Review
- Chemotherapy for Colorecal Cancer