J Korean Med Sci.
2013 Oct;28(10):1549-1551. 10.3346/jkms.2013.28.10.1549.
Secondary Prophylaxis of Docetaxel Induced Diarrhea with Loperamide: Case Report
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, St. Vincent's Hospital, The Catholic University of Korea, Suwon, Korea. miongsok@catholic.ac.kr
- KMID: 1777697
- DOI: http://doi.org/10.3346/jkms.2013.28.10.1549
Abstract
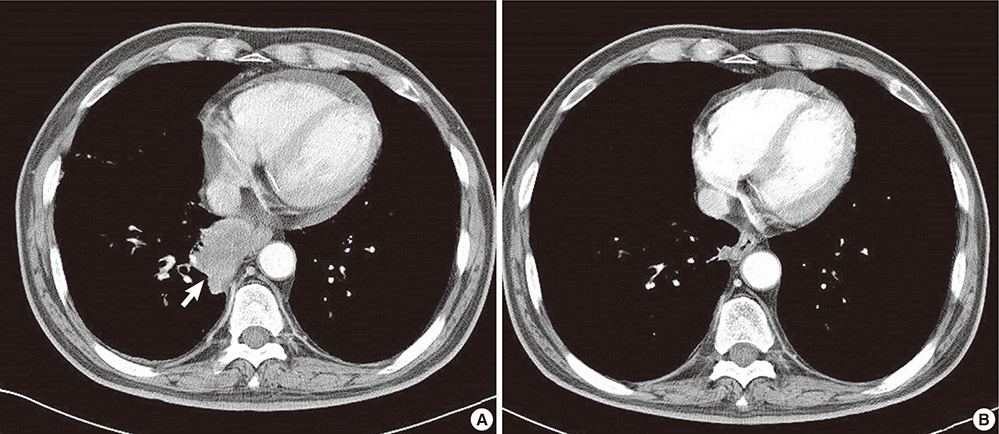
- Diarrhea is a common adverse event of docetaxel with 20%-40% of incidence and severe diarrhea occurs in 5%-6%. Several treatment guidelines for chemotherapy induced diarrhea (CID) exist, however the prophylaxis for that is not well known. We describe a new prophylactic approach for the CID with loperamide. A 72-yr-old male patient with stage IV non-small-cell lung cancer developed diarrhea repeatedly after docetaxel-cisplatin chemotherapy. His diarrhea persisted despite treatment including loperamide and fasting. However, the diarrhea was successfully prevented when loperamide was given before and after the chemotherapy. To our knowledge, this is the first report of prophylactic approach for the CID with loperamide.
Keyword
MeSH Terms
-
Aged
Carcinoma, Non-Small-Cell Lung/*drug therapy/radiography
Cisplatin/therapeutic use
Diarrhea/chemically induced/*etiology
Drug Therapy, Combination
Humans
Loperamide/*adverse effects/therapeutic use
Lung Neoplasms/*drug therapy/radiography
Male
Neoplasm Staging
Taxoids/*adverse effects/therapeutic use
Tomography, X-Ray Computed
Cisplatin
Loperamide
Taxoids
Figure
Reference
-
1. Richardson G, Dobish R. Chemotherapy induced diarrhea. J Oncol Pharm Pract. 2007; 13:181–198.2. Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol. 2010; 2:51–63.3. Benson AB 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA Jr, McCallum R, Mitchell EP, O'Dorisio TM, Vokes EE, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004; 22:2918–2926.4. Kornblau S, Benson AB, Catalano R, Champlin RE, Engelking C, Field M, Ippoliti C, Lazarus HM, Mitchell E, Rubin J, et al. Management of cancer treatment-related diarrhea: issues and therapeutic strategies. J Pain Symptom Manage. 2000; 19:118–129.5. Eisenhauer EA, Vermorken JB. The toxoids: comparative clinical pharmacology and therapeutic potential. Drugs. 1998; 55:5–30.6. DailyMed. TAXOTERE (docetaxel) injection, solution, concentrate [sanofi-aventis U.S. LLC]. accessed on 3 January 2014. Available at http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82731db6-92fc-483b-9d73-9b2aed79b104.7. Saltz LB. Understanding and managing chemotherapy-induced diarrhea. J Support Oncol. 2003; 1:35–46.8. Wardill HR, Bowen JM, Gibson RJ. Chemotherapy-induced gut toxicity: are alterations to intestinal tight junctions pivotal? Cancer Chemother Pharmacol. 2012; 70:627–635.9. Ericsson CD, Johnson PC. Safety and efficacy of loperamide. Am J Med. 1990; 88:10S–14S.10. Kirby MG, Dukes GE, Heizer WD, Bryson JC, Powell JR. Effect of metoclopramide, bethanechol, and loperamide on gastric residence time, gastric emptying, and mouth-to-cecum transit time. Pharmacotherapy. 1989; 9:226–231.11. Anthony L. New strategies for the prevention and reduction of cancer treatment-induced diarrhea. Semin Oncol Nurs. 2003; 19:17–21.12. Carrion AF, Hosein PJ, Cooper EM, Lopes G, Pelaez L, Rocha-Lima CM. Severe colitis associated with docetaxel use: a report of four cases. World J Gastrointest Oncol. 2010; 2:390–394.13. Stemmler HJ, Kenngotte S, Diepolder H, Heinemann V. Gastrointestinal toxicity associated with weekly docetaxel treatment. Ann Oncol. 2002; 13:978–981.14. Sezer O, Eucker J, Possinger K. Colitis associated with docetaxel-based chemotherapy. Lancet. 2000; 355:1823–1824.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Loperamide Oxide in Acute Diarrhea in Adults : A Randomized Double-Blind Placebo-controlled Multicenter Trial
- Pharmacologic Agents for Chronic Diarrhea
- The Effect of Probiotic on Constipation in Rats
- A Case of Localized Erythematous Dermatitis on the Site of a Docetaxel Injection
- Metabolomics approach to serum biomarker for loperamide-induced constipation in SD rats