Yonsei Med J.
2009 Dec;50(6):832-837. 10.3349/ymj.2009.50.6.832.
The Protective Effect of Sodium Hyaluronate on the Cartilage of Rabbit Osteoarthritis by Inhibiting Peroxisome Proliferator-Activated Receptor-Gamma Messenger RNA Expression
- Affiliations
-
- 1Department of Orthopedics, Renmin Hospital of Wuhan University, Wuhan, Hubei, China. zhoujianlin1688@yahoo.com.cn
- 2Department of Respiratory Medicine, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Key Laboratory of Respiratory Disease of Ministry of Public Health, Wuhan, Hubei, China.
- KMID: 1777099
- DOI: http://doi.org/10.3349/ymj.2009.50.6.832
Abstract
- PURPOSE
The purpose of this study was to study the protective effect and influence of sodium hyaluronate (Na-HA) on mRNA expression of peroxisome proliferators-activated receptor gamma (PPAR-gamma) in cartilage of rabbit osteoarthritis (OA) model.
MATERIALS AND METHODS
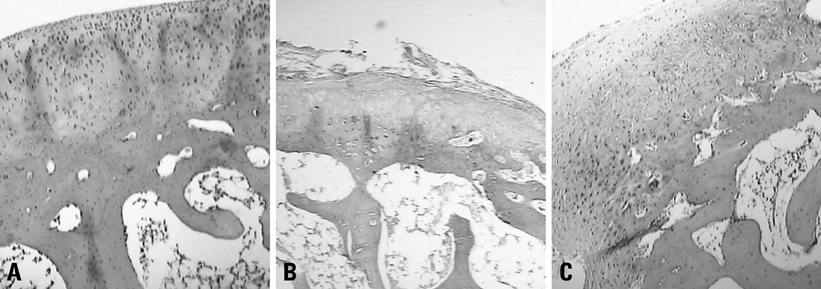
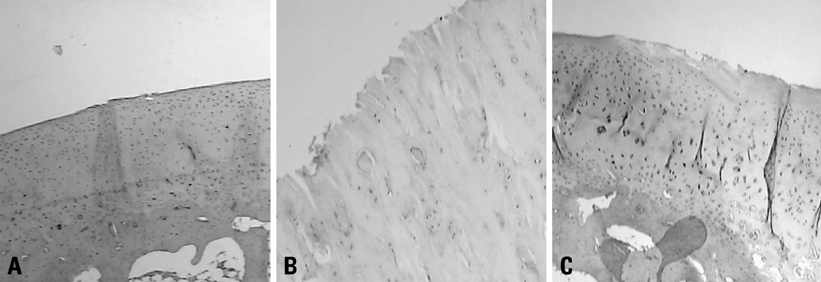
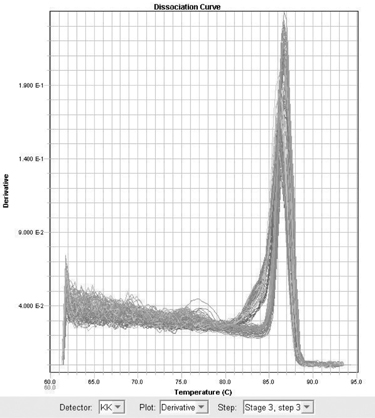
Forty eight white rabbits were randomly divided into A, B, and C groups. Group A was normal control group, B and C groups underwent unilateral anterior cruciate ligament transection (ACLT). The rabbits in group B were injected normal saline after ACLT; and Group C received intraarticular1% sodium hyaluronate (HA) injection 5 weeks after surgery, 0.3 mL once a week. At 11th week after surgery, all the rabbits were sacrificed. The cartilage changes on the medial femoral condyles were graded separately. Cartilage sections were stained with safranin-O and HE, and messenger RNA (mRNA) expression of PPAR-gamma was detected by using real time polymerase chain reaction (Real Time-PCR).
RESULTS
Cartilage degeneration in group B was significantly more severe than in A and C injection group. The grey value of Safranin-O of B group was higher than A and C groups. Expression of PPAR-gamma mRNA in group B was higher than group A and C.
CONCLUSION
This study shows that Na-HA has a protective effect on articular cartilage degeneration, and the inhibitory effect on the PPAR-gamma mRNA expression may be one of therapeutic mechanism of Na-HA.
Keyword
MeSH Terms
-
Animals
Cartilage/*drug effects/*metabolism
Gene Expression/drug effects/genetics
Hyaluronic Acid/*pharmacology/therapeutic use
Microscopy
Osteoarthritis/*drug therapy/metabolism
PPAR gamma/*genetics
RNA, Messenger/*genetics
Rabbits
Random Allocation
Reverse Transcriptase Polymerase Chain Reaction
Viscosupplements/*pharmacology/therapeutic use
Figure
Reference
-
1. Studer R, Jaffurs D, Stefanovic-Racic M, Robbins PD, Evans CH. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999. 7:377–379.
Article2. Hashimoto S, Setareh M, Ochs RL, Lotz M. Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum. 1997. 40:1749–1755.
Article3. Kühn K, Hashimoto S, Lotz M. Cell density modulates apoptosis in human articular chondrocytes. J Cell Physiol. 1999. 180:439–447.
Article4. Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res. 2001. 19:785–796.
Article5. Shan ZZ, Masuko-Hongo K, Dai SM, Nakamura H, Kato T, Nishioka K. A Potential role of 15-deoxy (12,14) prostaglandin J2 for induction of human articular chondrocyte apoptosis in arthritis. J Biol Chem. 2004. 279:37939–37950.
Article6. Liu SQ, Qiu B, Chen LY, Peng H, Du YM. The effects of carboxymethylated chitosan on metalloproteinase-1, -3 and tissue inhibitor of metalloproteinase-1 gene expression in cartilage of experimental osteoarthritis. Rheumatol Int. 2005. 26:52–57.
Article7. Muir H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays. 1995. 17:1039–1048.
Article8. Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998. 41:1632–1638.
Article9. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001. 344:907–916.
Article10. Roach HI, Aigner T, Kouri JB. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis. 2004. 9:265–277.11. Feng L, Precht P, Balakir R, Horton WE Jr. Evidence of a direct role for Bcl-2 in the regulation of articular chondrocyte apoptosis under the conditions of serum withdrawal and retinoic acid treatment. J Cell Biochem. 1998. 71:302–309.
Article12. Miwa M, Saura R, Hirata S, Hayashi Y, Mizuno K, Itoh H. Induction of apoptosis in bovine articular chondrocyte by prostaglandin E(2) through cAMP-dependent pathway. Osteoarthritis Cartilage. 2000. 8:17–24.
Article13. Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996. 1302:93–109.
Article14. Carlberg C, Wiesenberg I. The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: an unexpected relationship. J Pineal Res. 1995. 18:171–178.
Article15. Bordji K, Grillasca JP, Gouze JN, Magdalou J, Schohn H, Keller JM, et al. Evidence for the presence of peroxisome proliferator-activated receptor (PPAR) alpha and gamma and retinoid Z receptor in cartilage. PPARgamma activation modulates the effects of interleukin-1beta on rat chondrocytes. J Biol Chem. 2000. 275:12243–12250.
Article16. Shao YY, Wang L, Hicks DG, Tarr S, Ballock RT. Expression and activation of peroxisome proliferator-activated receptors in growth plate chondrocytes. J Orthop Res. 2005. 23:1139–1145.
Article17. Yoon EK, Lee WK, Lee JH, Yu SM, Hwang SG, Kim SJ. ERK-1/-2 and p38 kinase oppositely regulate 15-deoxy-delta (12,14)-prostaglandinJ2-Induced PPAR-gamma activation that mediates dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J Korean Med Sci. 2007. 22:1015–1021.
Article18. Yasui T, Akatsuka M, Tobetto K, Hayaishi M, Ando T. The effect of hyaluronan on interleukin-1 alpha-induced prostaglandin E2 production in human osteoarthritic synovial cells. Agents Actions. 1992. 37:155–156.
Article19. Tobetto K, Yasui T, Ando T, Hayaishi M, Motohashi N, Shinogi M, et al. Inhibitory effects of hyaluronan on [14C]arachidonic acid release from labeled human synovial fibroblasts. Jpn J Pharmacol. 1992. 60:79–84.
Article20. Larsen NE, Lombard KM, Parent EG, Balazs EA. Effect of hylan on cartilage and chondrocyte cultures. J Orthop Res. 1992. 10:23–32.
Article21. Presti D, Scott JE. Hyaluronan-mediated protective effect against cell damage caused by enzymatically produced hydroxyl (OH.) radicals is dependent on hyaluronan molecular mass. Cell Biochem Funct. 1994. 12:281–288.
Article22. Campo GM, Avenoso A, Campo S, Ferlazzo AM, Altavilla D, Calatroni A. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther. 2003. 5:R122–R131.23. Sasaki A, Sasaki K, Konttinen YT, Santavirta S, Takahara M, Takei H, et al. Hyaluronate inhibits the interleukin-1beta-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J Exp Med. 2004. 204:99–107.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Downregulation of Peroxisome Proliferator-Activated Receptor (PPAR)alpha, PPARgamma, and Phosphoglycerate Mutase 2 in Prostate Cancer
- The Effect of Chlamydia pneumoniae on the Expression of Peroxisome Proliferator-Activated Receptor-gamma in Vascular Smooth Muscle Cells
- Peroxisome Proliferator-Activated Receptor gammaActivation Promotes Adipogenesis in Human Mesenchymal Stem Cells
- Sterol-independent repression of low density lipoprotein receptor promoter by peroxisome proliferator activated receptor gamma coactivator-1alpha (PGC-1alpha)
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy