Korean J Gastroenterol.
2010 Jul;56(1):39-44. 10.4166/kjg.2010.56.1.39.
Successful Resection of Locally Advanced Gastrointestinal Stromal Tumor of the Ampulla of Vater after Treatment with Imatinib
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, School of Medicine, Kyung Hee University, Seoul, Korea. gidrdong@chol.com
- KMID: 1775877
- DOI: http://doi.org/10.4166/kjg.2010.56.1.39
Abstract
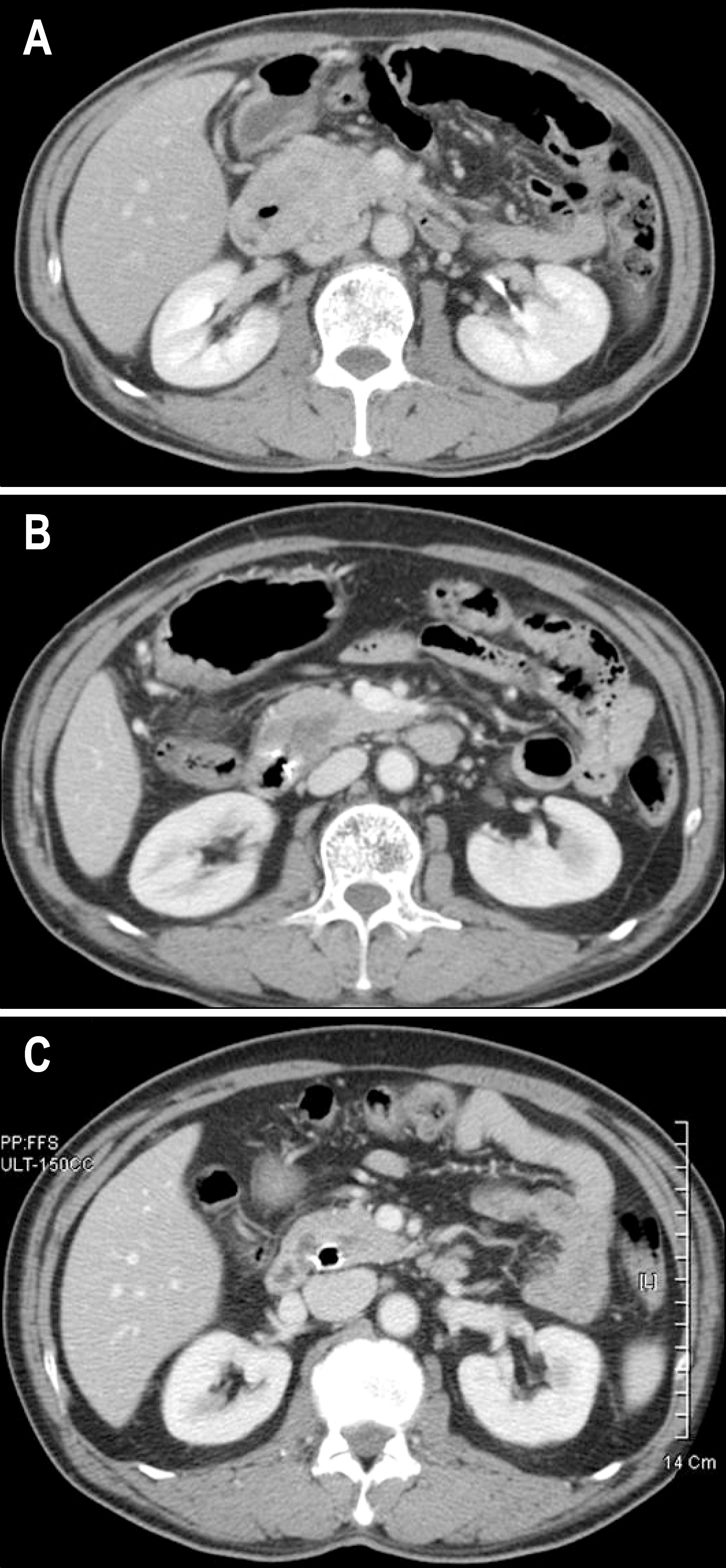
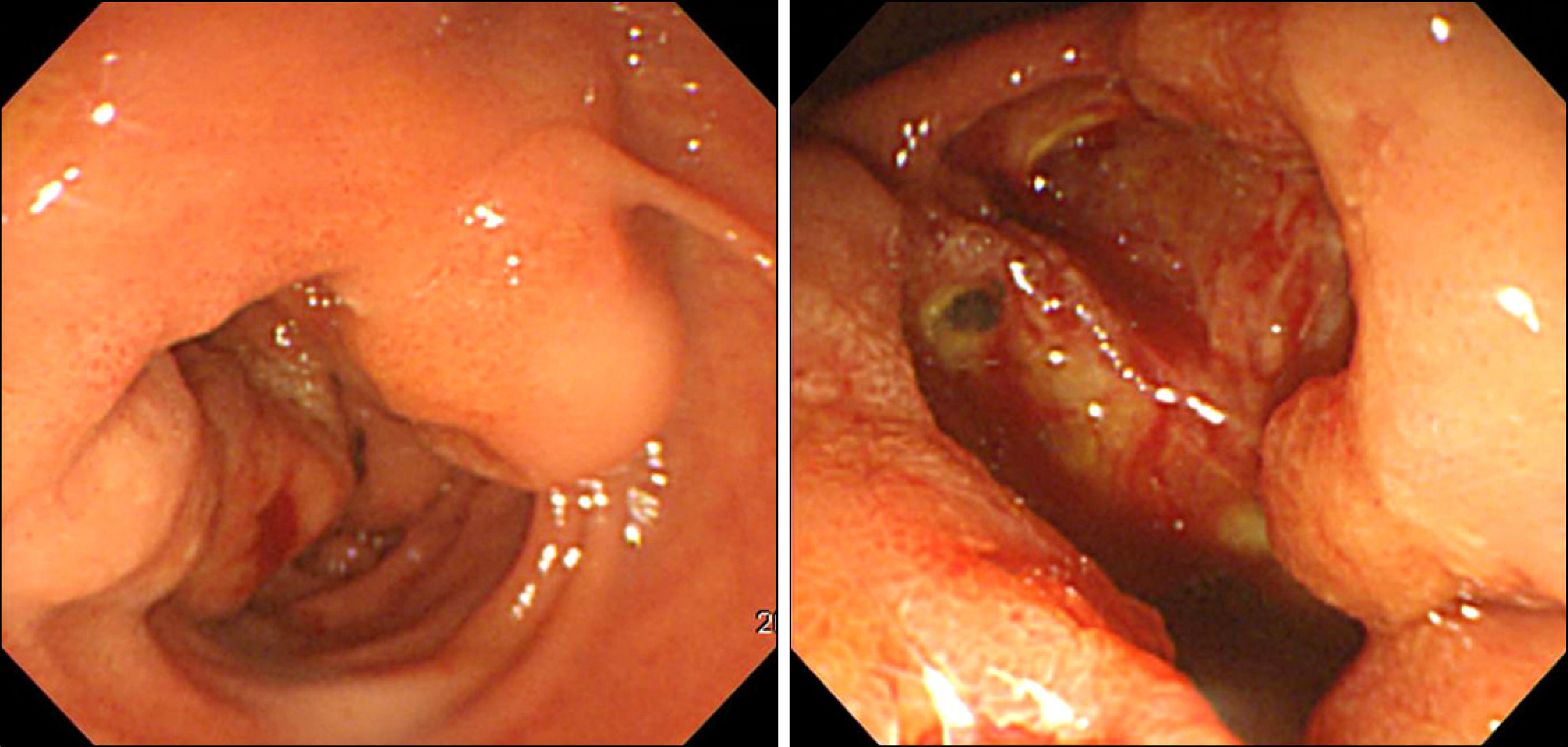
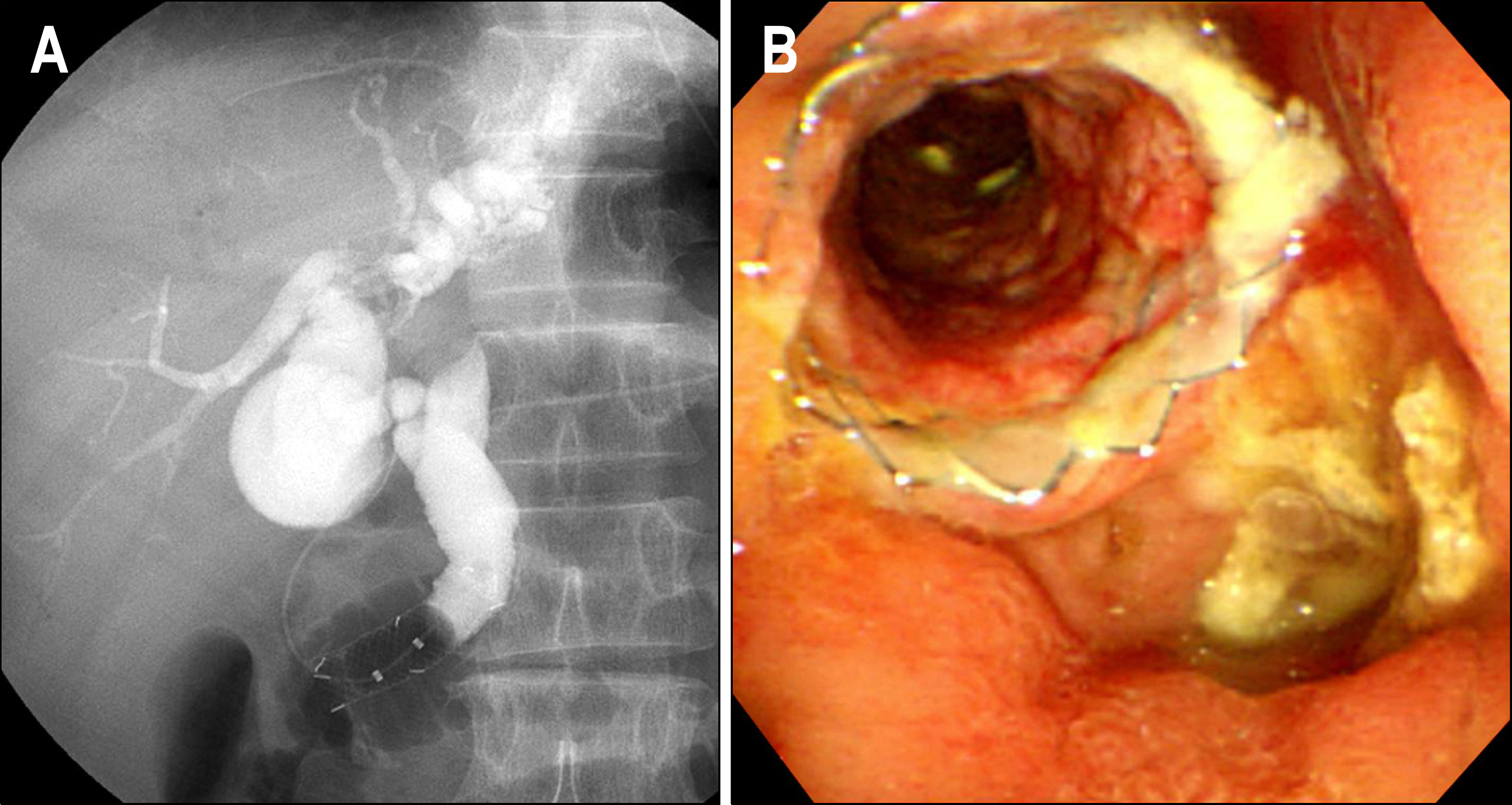
- Gastrointestinal stromal tumor (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract arising from Cajal's cells, expressing CD 117. The standard treatment for primary GIST is complete surgical resection. Imatinib mesylate, a specific tyrosine kinase inhibitor, is effective against locally advanced and metastatic GIST. There are several reports of the effect of preoperative imatinib in patients with unresectable and locally advanced primary GIST. We report a case of unresectable primary GIST of the ampulla of Vater, which we were able to completely resect after treatment with a dosage of imatinib 400 mg daily for 5 months. Twelve months later, the patient was treated with imatinib and doing well with no evidence of recurrence.
MeSH Terms
Figure
Reference
-
1. Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000; 7:705–712.
Article2. Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999; 30:1213–1220.
Article3. Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998; 152:1259–1269.4. Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiq-uitous feature of gastrointestinal stromal tumors. Cancer Res. 2001; 61:8118–8121.5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–465.
Article6. Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors: report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005; 16:566–578.
Article7. Choi WH, Kim S, Hyung WJ, et al. Long-surviving patients with recurrent GIST after receiving cytoreductive surgery with imatinib therapy. Yonsei Med J. 2009; 50:437–440.
Article8. Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999; 23:82–87.9. Greenson JK. Gastrointestinal stromal tumors and other mesenchymal lesions of the gut. Mod Pathol. 2003; 16:366–375.
Article10. Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23:70–83.
Article11. Yang WL, Yu JR, Wu YJ, et al. Duodenal gastrointestinal stromal tumor: clinical, pathologic, immunohistochemical characteristics, and surgical prognosis. J Surg Oncol. 2009; 100:606–610.
Article12. Kim SH, Kim JH, Baik GH, et al. Malignant gastrointestinal stromal tumor of the ampulla of Vater: a case report. Korean J Gastroenterol. 2004; 43:66–70.13. Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006; 244:176–184.14. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006; 130:1466–1478.
Article15. Demetti GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–480.
Article16. Andtbacka RH, Ng CS, Scaife CL, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol. 2007; 14:14–24.
Article17. Fiore M, Palassini E, Fumagalli E, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol. 2009; 35:739–745.
Article18. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose Imatinib: randomized trial. Lancet. 2004; 364:1127–1134.19. Haller F, Detken S, Schulten HJ, et al. Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann Surg Oncol. 2007; 14:526–532.
Article20. Bü mming P, Andersson J, Meis-Kindblom JM, et al. Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours (GIST) with imatinib: a centre-based study of 17 patients. Br J Cancer. 2003; 89:460–464.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Imatinib in Locally Advanced Gastrointestinal Stromal Tumors of the Stomach: Report of Three Cases
- Esophagus, Stomach & Intestine; A Case of Malignant Carcinoid Tumor of the Ampulla of Vater
- Successful Endoscopic Resection of a Rectal Gastrointestinal Stromal Tumor Larger Than 5 cm
- Efficacy of Imatinib Mesylate Neoadjuvant Treatment for a Locally Advanced Rectal Gastrointestinal Stromal Tumor
- Imatinib-induced DRESS Syndrome in Gastrointestinal Stromal Tumor