Yonsei Med J.
2009 Jun;50(3):352-357. 10.3349/ymj.2009.50.3.352.
Correlation of Post Swim-Up Acrosome Index with in-vitro Fertilization Outcomes
- Affiliations
-
- 1Department of Histology-Embryology, University of Suleyman Demirel, Medical School, Isparta, Turkey. mozguner@hotmail.com
- 2Department of Histology-Embryology, University of Ankara, Medical School, Ankara, Turkey.
- 3Department of Obstetrics and Gynaecology, University of Suleyman Demirel, Medical School, Isparta, Turkey.
- KMID: 1758562
- DOI: http://doi.org/10.3349/ymj.2009.50.3.352
Abstract
-
PURPOSE: A prospective study was planned to determine the relationship between post swim-up acrosome index (AI) evaluation and fertilization outcomes in an in vitro fertilization (IVF) program.
MATERIALS AND METHODS
Infertile couples who have applied to IVF were admitted into this study when the male partner's sperm concentration was > 20x106/mL and motility > 30%. Pre- and post swim-up semen quality parameters including concentration, motility, sperm morphology and AI were evaluated in a prospective, randomized and blinded fashion. The couples were divided prospectively into 2 groups. In group I (25 couples) 50 000 sperm per oocyte were used for insemination considering post swim-up acrosome index, and in group II (25 couples) 50 000 sperm per oocyte were used for insemination without considering post swim-up acrosome index.
RESULTS
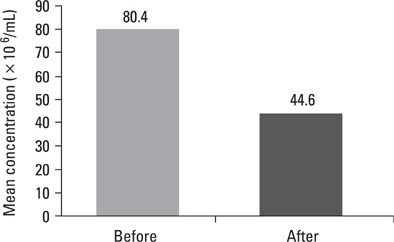
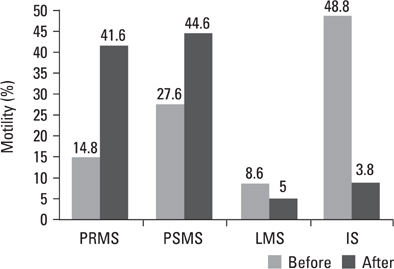
Pre- and post swim-up AI were 30.8 +/- 3.4 and 17.8 +/- 4.5 in group I, and 31.4 +/- 3.6 and 16.3 +/- 4.7 in group II (p > 0.05) respectively. The significant improvement in morphology and motility after double wash swim-up procedure has been observed. However, double wash swim-up procedure could not eliminate head and especially acrosomal defects which would directly effect fertilization capacity in conventional IVF program. In group I, 85.3% of oocytes were fertilized, with a 48% pregnancy rate; in group II, 71.0% of oocytes were fertilized, with a pregnancy rate of 20%. Fertilization and pregnancy rates were significantly different (p < 0.05) between the two groups.
CONCLUSION
We have concluded that it could be useful to consider post swim-up AI of sperm inseminated in conventional IVF cycles, which correlates with high fertilization and pregnancy rates.
MeSH Terms
Figure
Reference
-
1. Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986. 46:1118–1123.
Article2. Mashiach R, Fisch B, Eltes F, Tadir Y, Ovadia J, Bartoov B. The relationship between sperm ultrastructural features and fertilizing capacity in vitro. Fertil Steril. 1992. 57:1052–1057.
Article3. Enginsu ME, Dumoulin JC, Pieters MH, Bras M, Evers JL, Geraedts JP. Evaluation of human sperm morphology using strict criteria after Diff-Quik staining: correlation of morphology with fertilization in vitro. Hum Reprod. 1991. 6:854–858.
Article4. Oehninger S, Mahony M, Ozgür K, Kolm P, Kruger T, Franken D. Clinical significance of human sperm-zona pellucida binding. Fertil Steril. 1997. 67:1121–1127.5. Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Veeck LL, et al. New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology. 1987. 30:248–251.
Article6. Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988. 49:112–117.
Article7. Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress and sperm function. J Androl. 1996. 17:276–287.8. Menkveld R, Rhemrev JP, Franken DR, Vermeiden JP, Kruger TF. Acrosomal morphology as a novel criterion for male fertility diagnosis: relation with acrosin activity, morphology (strict criteria) and fertilization in vitro. Fertil Steril. 1996. 65:637–644.
Article9. Francavilla F, Romano R, Santucci R, Poccia G. Effect of sperm morphology and motile sperm count on outcome of intrauterine insemination in oligozoospermia and/or asthenozoospermia. Fertil Steril. 1990. 53:892–897.
Article10. Sanchezn Sarmiento CA, Coetzee K, Kruger TF, van der Merwe JP, Stander FS, et al. Comparison between swim-up and glass wool column filtration of human semen in a gamete intrafallopian transfer program. Arch Androl. 1996. 36:155–160.
Article11. Scott RT Jr, Oehninger SC, Menkveld R, Veeck LL, Acosta AA. Critical assessment of sperm morphology before and after double wash swim-up preparation for in vitro fertilization. Arch Androl. 1989. 23:125–129.
Article12. Lopata A, Patullo MJ, Chang A, James B. A method for collecting motile spermatozoa from human semen. Fertil Steril. 1976. 27:677–684.
Article13. Paulson JD, Polakoski KL. A glass wool column procedure for removing extraneous material from the human ejaculate. Fertil Steril. 1977. 28:178–181.
Article14. Gorus FK, Pipeleers DG. A rapid method for the fractionation of human spermatozoa according to their progressive motility. Fertil Steril. 1981. 35:662–665.
Article15. World Health Organisation. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 1992. 3rd ed. Cambridge: Cambridge University Press.16. Duncan WW, Glew MJ, Wang XJ, Flaherty SP, Matthews CD. Prediction of in vitro fertilization rates from semen variables. Fertil Steril. 1993. 59:1233–1238.17. Kobayashi T, Jinno M, Sugimura K, Nozawa S, Sugiyama T, Iida E. Sperm morphological assessment based on strict criteria and in-vitro fertilization outcome. Hum Reprod. 1991. 6:983–986.18. Enginsu ME, Pieters MH, Dumoulin JC, Evers JL, Geraedts JP. Male factor as determinant of in-vitro fertilization outcome. Hum Reprod. 1992. 7:1136–1140.19. Morgentaler A, Fung MY, Harris DH, Powers RD, Alper MM. Sperm morphology and in vitro fertilization outcome: a direct comparison of World Health Organization and strict criteria methodologies. Fertil Steril. 1995. 64:1177–1182.
Article20. McDowel JS, Veeck LL, Jones HW Jr. Analysis of human spermatozoa before and after processing for in vitro fertilization. J In Vitro Fert Embryo Transf. 1985. 2:23–26.
Article21. Mashiach R, Fisch B, Eltes F, Tadir Y, Ovadia J, Bartoov B. The relationship between sperm ultrastructural features and fertilizing capacity in vitro. Fertil Steril. 1992. 57:1052–1057.
Article22. Bartoov B, Eltes F, Langsam J, Snyder M, Fisher J. Structural studies in morphological assessment of human spermatozoa. Int J Androl. 1982. 5:81–96.
Article23. Jeulin C, Feneux D, Serres C, Jouannet P, Guillet-Rosso F, Belaisch-Allart J, et al. Sperm factors related to failure of human in-vitro fertilization. J Reprod Fertil. 1986. 76:735–744.
Article24. Rhemrev JP, Menkveld R, Roseboom TJ, van Overveld FW, Teerlink T, Lombard C, et al. The acrosome index, radical buffer capacity and number of isolated progressively motile spermatozoa predict IVF results. Hum Reprod. 2001. 16:1885–1892.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Fertilization Promoting Peptide (FPP) on the Acrosome Status of Cryopreserved Human Sperm
- Effect of Fertilization Promoting Peptide on Kinematic Parameters, Capacitaion and Acrosome Reaction in Human Spermatozoa
- A study about fertilization rate following reinsemination in in vitro fertilization
- Percentage of motile spermatozoa at 22 hours after swim-up procedure: An indicator for intracytoplasmic sperm injection?
- Ca2+-ATPase Role in the Capacitation and Acrosome Reaction Assessed by a Chlortetracycline Fluorescence Assay