Cancer Res Treat.
2005 Feb;37(1):44-47.
Comparison of 30 mg and 40 mg of Mitomycin C Intravesical Instillation in Korean Superficial Bladder Cancer Patients: Prospective, Randomized Study
- Affiliations
-
- 1Department of Urology, Seoul National University College of Medicine, Seoul, Korea. selee@snubh.org
Abstract
- PURPOSE
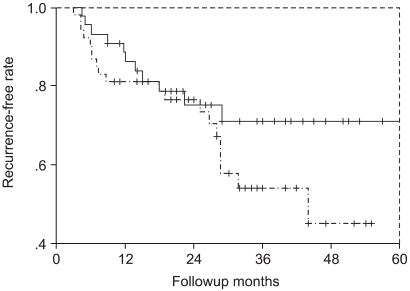
A prospective study was performed to compare the efficacy and safety of intravesical mitomycin C (MMC) instillation for the prophylaxis of bladder cancer at different concentrations (30 mg or 40 mg). MATERIALS AND METHODS: Ninety-seven patients that received complete transurethral resection for superficial bladder cancer were divided into two-randomized groups. One group (n=53) received 30 mg and the other group (n=44) received 40 mg dose of MMC weekly for 8 weeks, which was followed monthly for 10 months as maintenance therapy. The recurrence rates and side effects in both groups were recorded. The mean follow-up period was 32.4 months in the 30 mg group, and 32.0 months in the 40 mg group. RESULTS: The overall one and two year recurrence rates were 19% and 24% in the 30 mg group, and 12% and 22% in the 40 mg group, which was not significantly different (p>0.05). Most of the side effects were mild and transient. Moreover, the rates of the individual side effects were not statistically different in the two groups. CONCLUSION: Our comparison of 30 mg and 40 mg intravesical MMC instillation showed no difference in either response or side effects. Thus, we tentatively conclude that we can use 30 mg instead of 40 mg as an intravesical MMC instillation dose.
Keyword
MeSH Terms
Figure
Reference
-
1. Lutzeyer W, Rubben H, Dahm H. Prognostic parameters in superficial bladder cancer: an analysis of 315 cases. J Urol. 1982; 127:250–252. PMID: 7062375.
Article2. Park CH, Kim CI, Lee SC. Prognostic indexes in patients treated with intravesical bacillus calmette - guerin for superficial bladder cancer. J Korean Cancer Assoc. 1991; 23:835–842.3. Utz DC, Hanash KA, Farrow GM. The plight of the patient with carcinoma in situ of the bladder. J Urol. 1970; 103:160–164. PMID: 5410591.
Article4. Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992; 19:573–580. PMID: 1636241.
Article5. Huland H, Otto U, Droese M, Kloppel G. Long-term mitomycin C instillation after transurethral resection of superficial bladder carcinoma: Influence on recurrence, progression and survival. J Urol. 1984; 132:27–29. PMID: 6427483.
Article6. Mishina T, Oda K, Murata S, Ooe H, Mori Y. Mitomycin C bladder instillation therapy for bladder tumors. J Urol. 1975; 114:217–219. PMID: 1159911.
Article7. Solsona E, Iborra I, Ricos JV, Monros JL, Casanova J, Dumont R. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol. 1999; 161:1120–1123. PMID: 10081851.
Article8. Badalament RA, Farah RN. Treatment of superficial bladder cancer with intravesical chemotherapy. Semin Surg Oncol. 1997; 13:335–341. PMID: 9259089.
Article9. Shelley MD, Wilt TJ, Court J, Coles B, Kynaston H, Mason MD. Intravesical bacillus Calmette-Guerin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int. 2004; 93:485–490. PMID: 15008714.
Article10. Lee ES, Kim HH, Min KJ, Lee C. A comparison of mitomycin C and bacillus calmette-guerin for the prophylaxis of high risk superficial bladder tumors. Korean J Urol. 1992; 33:1014–1019.11. Lee JH, Lee YG. A comparison of mitomycin C, pasteur strain bcg and tice strain bcg for the prophylaxis of superficial bladder tumor. Korean J Urol. 1997; 38:945–950.12. Son KW, Kim HS, Oh TH. Prophylactic effect of mitomycin C and bacillus calmette-guerin in stage t1 of the superficial bladder cancer. Korean J Urol. 1997; 38:957–962.13. Koontz WW Jr, Prout GR Jr, Smith W, Frable WJ, Minnis JE. The use of intravesical thio-tepa in the management of non-invasive carcinoma of the bladder. J Urol. 1981; 125:307–312. PMID: 6782251.
Article14. Masters JR, Popert RJ, Thompson PM, Gibson D, Coptcoat MJ, Parmar MK. Intravesical chemotherapy with epirubicin: a dose response study. J Urol. 1999; 161:1490–1493. PMID: 10210379.
Article15. Kondas J, Kiss L, Hatar A, Kiss A, Lukacs T, Szeldeli P, et al. The effect of intravesical mitomycin C on the recurrence of superficial (Ta-T1) bladder cancer. A Hungarian Multicenter Study. Int Urol Nephrol. 1999; 31:451–456. PMID: 10668939.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravesical Mitomycin C Instillation as a Prophylactic Treatment of Superficial Bladder Cancer
- Prophylactic Effect of Mitomycin C and Bacillus Calmette-Guerin in Stage T1 of the Superficial Bladder Cancer
- Prophylactic Effectiveness of Intravesical Mitomycin C Instillation to Prevent Recurrence of Superficial Bladder Carcinoma after Transurethral Resection
- A Comparative Clinical Study of Prophylatic Effect of Intravesical Instillation with Thiotepa and Mitomycin C after TUR for Superficial Bladder Tumor
- Effects of Intravesical Mitomycin-C on Bladder Cancer of Rats Induced by N-butyl-N-(4-Hydroxybutyl) Nitrosamine