J Korean Med Sci.
2004 Oct;19(5):744-749. 10.3346/jkms.2004.19.5.744.
Proton Magnetic Resonance Spectroscopic Changes of the Primary Motor Cortex and Supplementary Motor Area in Hemiparetic Patients with Corticospinal Tract Injury due to Deep Intracerebral Hematoma
- Affiliations
-
- 1Department of Neurosurgery, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea. sbc@catholic.ac.kr
- 2Department of Biomedical Engineering, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
- KMID: 1733520
- DOI: http://doi.org/10.3346/jkms.2004.19.5.744
Abstract
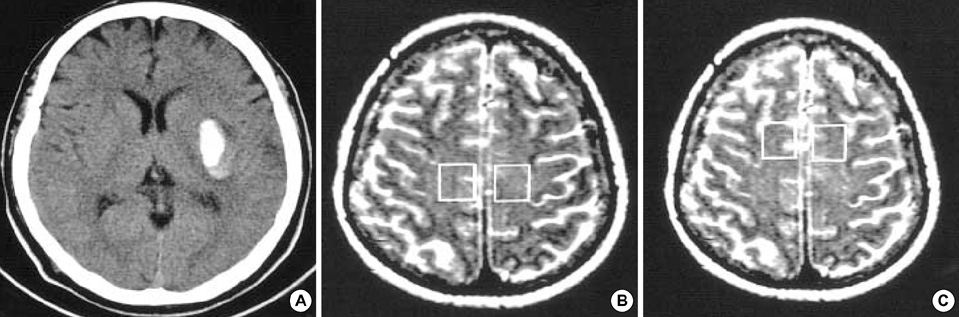
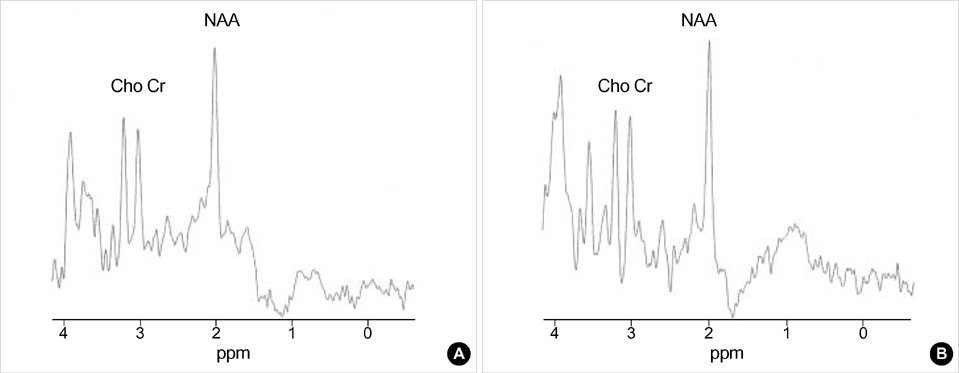
- This study was conducted to investigate the metabolic changes in the motor and motor association cortices following axonal injury in the internal capsule that was caused by deep intracerebral hematoma. Using proton magnetic resonance spectroscopy (1H MRS), the authors studied the primary motor cortices (M-1) and sup-plementary motor areas (SMA) of 9 hemiparetic patients with documentable hemi-paresis of varying severity, and we studied 10 normal volunteers as controls. To measure the M-1 and SMA biochemical changes, 4 separate single volumes of inter-est(VOIs) were located bilaterally in the affected and unaffected hemisphere (AH and UH).1H MRS provided a neuronal and axonal viability index by measuring levels of N-acetylaspartate (NAA) and creatine/phosphocreatine (Cr). The M-1/SMA NAA/Cr ratios of the AH and UH in patients, and the AH and normal volunteers were com-pared. The NAA/Cr ratios of the M-1 and SMA in AH, and the SMA in UH were sig-nificantly lower than those of normal volunteers. These 1H MRS findings indicate that axonal injury in the descending motor pathway at the level of internal capsule could induce metabolic changes in the higher centers of the motor pathway.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Aspartic Acid/*analogs & derivatives/metabolism
Basal Ganglia Hemorrhage/metabolism/*pathology
Creatine/metabolism
Female
Humans
*Magnetic Resonance Spectroscopy
Male
Middle Aged
Motor Cortex/metabolism/*pathology
Paresis/metabolism/*pathology
Phosphocreatine/metabolism
Protons
Pyramidal Tracts/metabolism/*pathology
Figure
Reference
-
1. Rudkin TM, Arnold DL. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol. 1999. 56:919–926.
Article2. Van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology. 1990. 176:509–515.
Article3. Puri BK, Smith HC, Cox IJ, Sargentoni J, Savic G, Maskill DW, Frankel HL, Ellaway PH, Davey NJ. The human motor cortex after incomplete spinal cord injury: an investigation using proton magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry. 1998. 65:748–754.
Article4. Berkelbach van der Sprenkel JW, Luyten PR, van Rijen PC, Tulleken CA, den Hollander JA. Cerebral lactate detected by regional proton magnetic resonance spectroscopy in a patient with cerebral infarction. Stroke. 1988. 19:1556–1560.
Article5. Bruhn H, Frahm J, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Cerebral metabolism in man after acute stroke: new observations using localized proton NMR spectroscopy. Magn Reson Med. 1989. 9:126–131.
Article6. Gideon P, Henriksen O, Sperling B, Christiansen P, Olsen TS, Jorgensen HS, Arlien-Soborg P. Early time course of N-acetyl aspartate, creatine and phosphocreatine, and compounds containing choline in the brain after acute stroke: a proton magnetic resonance spectroscopy study. Stroke. 1992. 23:1566–1572.7. Federico F, Simone IL, Lucivero V, Giannini P, Laddomada G, Mezzapesa DM, Tortorella C. Prognostic value of proton magnetic resonance spectroscopy in ischemic stroke. Arch Neurol. 1998. 55:489–494.
Article8. Ford CC, Griffey RH, Matwiyoff NA, Rosenberg GA. Multivoxel 1H-MRS of stroke. Neurology. 1992. 42:1408–1412.9. Gideon P, Sperling B, Arlien-Soborg P, Olsen TS, Henriksen O. Long-term follow-up of cerebral infarction patients with proton magnetic resonance spectroscopy. Stroke. 1994. 25:967–973.
Article10. Graham GD, Kalvach P, Blamire AM, Brass LM, Fayad PB, Prichard JW. Clinical correlates of proton magnetic resonance spectroscopy findings after acute cerebral infarction. Stroke. 1995. 26:225–229.
Article11. Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: Initial applications to human brain in vivo. Magn Reson Med. 1989. 9:79–93.12. Frahm J, Merbold KD, Hanicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson. 1987. 72:502–508.
Article13. Kreis R, Farrow N, Ross BD. Localized 1H NMR spectroscopy in patients with chronic hepatic encephalopathy: Analysis of changes in cerebral glutamine, choline, and inositols. NMR Biomed. 1991. 4:109–116.14. Petroff OA, Spencer DD, Alger JR, Prichard JW. High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology. 1989. 39:1197–1202.
Article15. Gresham GE, Duncan PW, Stason WB. Post-stroke Rehabilitation. 1995. Rocksville, MD: U.S. Department of Health and Human Services. Public Health Services, Agency for Health Care Policy and Research.16. Tallan HH, Moore S, Stein WH. N-acetyl-L-aspartic acid in brain. J Biol Chem. 1956. 219:257–264.
Article17. Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetyl aspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors and immature oligodendrocytes in vitro. J Neurochem. 1992. 59:55–61.18. Ross B, Michaelis T. Clinical applications of magnetic resonance spectroscopy. Magn Reson Q. 1994. 10:191–247.19. De Stefano N, Matthews PM, Arnold DL. Reversible decrease in N-acetylaspartate after acutre brain injury. Magn Reson Med. 1995. 34:721–727.20. Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996. 7:1397–1400.21. Pendlebury ST, Blaire AM, Lee MA, Styles P, Matthews PM. Axonal injury in the internal capsule correlates with motor impairment after stroke. Stroke. 1999. 30:956–962.
Article22. Fu L, Matthews PM, De Stefano N, Worsley KJ, Narayanan S, Francis GS, Antel JP, Wolfson C, Arnold DL. Imaging axonal damage of normal-appearing white matter in multiple sclerosis. Brain. 1998. 121:103–113.
Article23. Pioro EP, Antel JP, Cashman NR, Arnold DL. Detection of cortical neuron loss in motor neuron disease by proton mangnetic resonance spectroscopic imaging in vivo. Neurology. 1994. 44:1933–1938.24. Husted CA, Goodin DS, Hugg JW, Maudsley AA, Tsuruda JS, de Bie SH, Fein G, Matson GB, Weiner MW. Biochemical alterations in multiple sclerosis lesions and normal appearing white matter detected by in vivo 31P and 1H spectroscopic imaging. Ann Neurol. 1994. 36:157–165.25. Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain recovery from striatocapsular infarction in man. Ann Neurol. 1992. 31:463–472.26. Jones EG. Jones EG, Peters A, editors. Connectivity of primate sensory-motor cortex. Cerebral cortex. 1986. Vol. 5. New York: Plenum Press;113–183.27. Wiesendanger M. Recent developments in studies of the supplementary motor area of primates. Rev Physiol Biochem Pharmacol. 1986. 103:1–59.
Article28. Marsden CD, Deecke L, Freund H-J, Hallet M, Passingham RE, Shibasaki H, Tanji J, Wiesendanger M. The functions of the supplementary motor area. Summary of a workshop. Adv Neurol. 1996. 70:477–487.29. Fries W, Danek A, Schneidtmann K, Hamburger C. Motor recovery following capsular stroke: role of descending pathways from multiple motor areas. Brain. 1993. 116:369–382.30. Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997. 28:2518–2527.
Article31. Medical Research Council. Memorandum no. 45. Aid to examination of the peripheral nervous system. 1976. London: Her Majesty's Stationery Office.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Applicability of Diffusion Tensor MR Imaging and Fiber Tractography That Predict Short-Term Functional Motor Outcome in Patients with Deep Intracerebral Hemorrhage
- Analysis of Corticospinal Tract Injury by Using the Diffusion Tensor Imaging of 3.0 T Magnetic Resonance in Patients with Hypertensive Intracerebral Hemorrhage
- Corticospinal Tract Compression by Hematoma in a Patient with Intracerebral Hemorrhage: A Diffusion Tensor Tractography and Functional MRI Study
- Cortical Activation Related to Motor and Sensory Tasks in Congenital Mirror Movement using Functional MRI
- Functional MR Imaging of the Motor Cortex in Active and Passive Movement: Qualitative and Quantitative Changes