J Vet Sci.
2009 Dec;10(4):293-297. 10.4142/jvs.2009.10.4.293.
Modification of pharmacokinetics of norfloxacin following oral administration of curcumin in rabbits
- Affiliations
-
- 1Department of Pharmacology and Toxicology, Veterinary College, Karnataka Veterinary, Animal & Fishery Sciences University, Postbox No.6, Bidar-585 401, India. pnadoor@rediffmail.com
- 2Department of Pharmacology & Toxicology, Veterinary College, Hebbal Campus, Bangalore-560 024, India.
- KMID: 1726900
- DOI: http://doi.org/10.4142/jvs.2009.10.4.293
Abstract
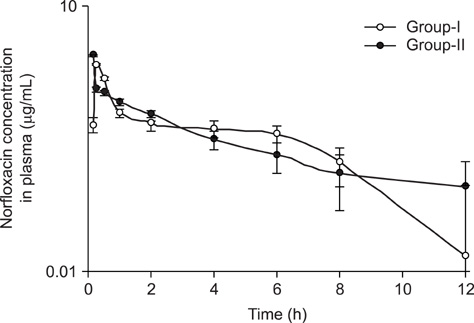
- Investigation was carried out in adult New Zealand white rabbits to study the influence of curcumin pre-treatment on pharmacokinetic disposition of norfloxacin following single oral administration. Sixteen rabbits were divided into two groups of eight each consisting of either sex. Animals in group-I were administered norfloxacin (100 mg/kg body weight p.o), while animals in group-II received similar dose of norfloxacin after pre-treatment with curcumin (60 mg/kg body weight per day, 3 days, p.o). Blood samples were drawn from the marginal ear vein into heparin-coated vials at 0 (zero time), 5, 10, 15, 30 min and 1, 2, 4, 6, 12 and 24 h post-treatment. Plasma norfloxacin concentrations were determined by high performance liquid chromatography. The plasma concentration-time profile of norfloxacin was adequately described by a one-compartment open model. The pharmacokinetic data revealed that curcumin-treated animals had significantly (p< or = 0.05) higher area under the plasma concentration-time curve and area under the first moment of plasma drug concentration-time curve. Prior treatment of curcumin significantly (p< or = 0.05) increased elimination half-life and volume of distribution of norfloxacin. Further treatment with curcumin reduced loading and maintenance doses by 26% and 24% respectively.
MeSH Terms
Figure
Reference
-
1. Anadón A, Martinez-Larrañaga MR, Velez C, Díaz MJ, Bringas P. Pharmacokinetics of norfloxacin and its N-desethyl- and oxo-metabolites in broiler chickens. Am J Vet Res. 1992. 53:2084–2089.2. Andersson MI, MacGowan AP. Development of the quinolones. J Antimicrob Chemother. 2003. 51:Suppl 1. 1–11.
Article3. Basak DN, Sarkar S, Chakrabarti A. Efficacy of norfloxacin: Nalidixic acid, chloramphenicol and furazolidone against canine haemorrhagic gastroenteritis. Indian Vet J. 1993. 70:263–264.4. Basu NK, Kole L, Kubota S, Owens IS. Human UDP-glucuronosyltransferases show atypical metabolism of mycophenolic acid and inhibition by curcumin. Drug Metab Disp. 2004. 32:768–777.
Article5. Chang ZQ, Oh BC, Kim JC, Jeong KS, Lee MH, Yun HI, Hwang MH, Park SC. Clinical Pharmacokinetics of norfloxacin-glycine acetate after intravenous and oral administration in pigs. J Vet Sci. 2007. 8:353–356.
Article6. Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: Biological actions and medicinal application. Curr Sci. 2004. 87:44–53.7. Dama MS, Varshneya C, Dardi MS, Katoch VC. Effect of trikatu pretreatment on the pharmacokinetics of pefloxacin administered orally in mountain Gaddi goats. J Vet Sci. 2008. 9:25–29.
Article8. Gibaldi M, Perrier D. Pharmacokinetics. 1982. 2nd ed. New York: Marcel Dekker;45–109.9. Lavy E, Ziv G, Glickman A. Intravenous disposition kinetics, oral and intramuscular bioavailability and urinary excretion of norfloxacin nicotinate in donkeys. J Vet Pharmacol Ther. 1995. 18:101–107.
Article10. Marcus S, Bernstein M, Ziv G, Glickman A, Gipps M. Norfloxacin nicotinate in the treatment of Pseudomonas aeruginosa infection in the genital tract of a bull. Vet Res Commun. 1994. 18:331–336.
Article11. Neuman M. Clinical pharmacokinetics of the newer antibacterial 4-quinolones. Clin Pharmacokinet. 1988. 14:96–121.
Article12. Notari RE. Biopharmaceutics and Clinical Pharmacokinetics: An Introduction. 1987. 4th ed. Marcel Dekker: New York;221–270.13. Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999. 27:486–494.14. Park SC, Yun HI, Oh TK. Comparative pharmacokinetic profiles of two norfloxacin formulations after oral administration in rabbits. J Vet Med Sci. 1988. 60:661–663.
Article15. Perl W, Samuel P. Input-output analysis for total input rate and total traced mass of body cholesterol in man. Circ Res. 1969. 25:191–199.
Article16. Rao GS, Ramesh S, Ahmad AH, Tripathi HC, Sharma LD, Malik JK. Effects of endotoxin-induced fever and probenecid on disposition of enrofloxacin and its metabolite ciprofloxacin after intravascular administration of enrofloxacin in goats. J Vet Pharmacol Ther. 2000. 23:365–372.
Article17. Rao TS, Basu N, Siddiqui HH. Anti-inflammatory activity of curcumin analogues. Indian J Med Res. 1982. 75:574–578.18. Ritschel WA. Handbook of Basic Pharmacokinetics. 1976. 3rd ed. Hamilton: Drug Intelligence;320–327.19. Rylander M, Norrby SR. Norfloxacin penetration into subcutaneous tissue cage fluid in rabbits and efficacy in vivo. Antimicrob Agents Chemother. 1983. 23:352–355.
Article20. Singh M, Varshneya C, Telang RS, Srivastava AK. Alteration of pharmacokinetics of oxytetracycline following oral administration of Piper longum in hens. J Vet Sci. 2005. 6:197–200.
Article21. Snedecor GW, Cochran WG. Statistical Methods. 1969. 6th ed. Ames: Iowa State University Press;59–65.22. Wolfson JS, Hooper DC. Norfloxacin: a new targeted fluoroquinolone antimicrobial agent. Ann Intern Med. 1988. 108:238–251.
Article23. Zhang W, Lim LY. Effects of spice constituents on P-glycoprotein-mediated transport and CYP3A4-mediated metabolism in vitro. Drug Metab Dispos. 2008. 36:1283–1290.
Article24. Zhang W, Tan TM, Lim LY. Impact of curcumin-induced changes in P-glycoprotein and CYP3A expression on the pharmacokinetics of peroral celiprolol and midazolam in rats. Drug Metab Dispos. 2007. 35:110–115.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical pharmacokinetics of norfloxacin-glycine acetate after intravenous and oral administration in pigs
- Treatment of Uncomplicated Male Gonococcal Urethritis with Norfloxacin
- Norfloxacin Hydrogel Coated Urethral Catheters for Prevention of Catheter Associated Urinary Tract Infection in the Rabbit
- Formulation and Evaluation of Irinotecan Suppository for Rectal Administration
- Pharmacokinetics of Uridine Following Ocular, Oral and Intravenous Administration in Rabbits