Yonsei Med J.
2006 Feb;47(1):93-104. 10.3349/ymj.2006.47.1.93.
Quantitative EMG Changes During 12-Week DeLorme's Axiom Strength Training
- Affiliations
-
- 1Department of Occupational Therapy, Kaya University, College of Health Science, Goryeong-gun, Gyeongbuk, Korea. hkshin1@kaya.ac.kr
- 2Graduate School of Rehabilitation Therapy, Department of Physical Therapy, Yonsei University Wonju Campus, College of Health Science, Wonju, Korea.
- 3Department of Rehabilitation Medicine, Yonsei University, Wonju College of Medicine, Wonju, Korea.
- KMID: 1715878
- DOI: http://doi.org/10.3349/ymj.2006.47.1.93
Abstract
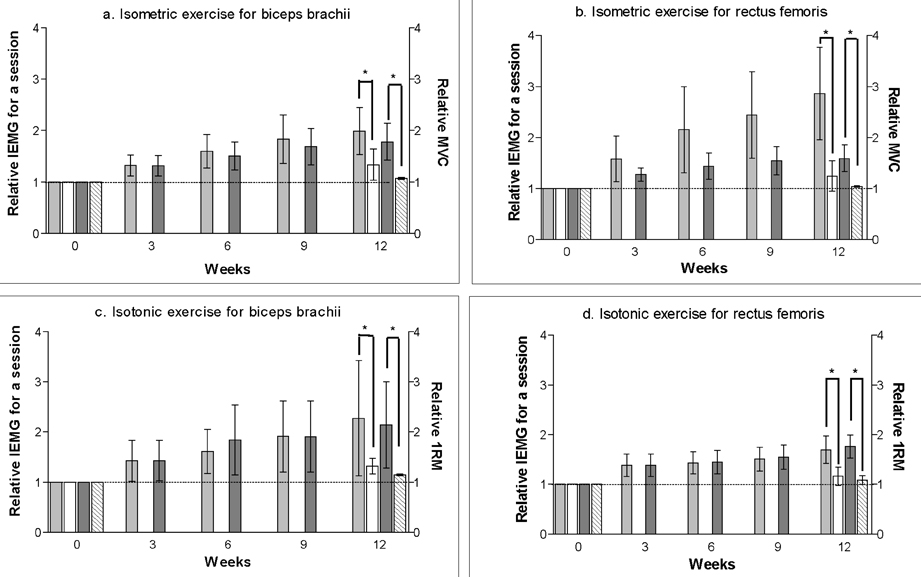
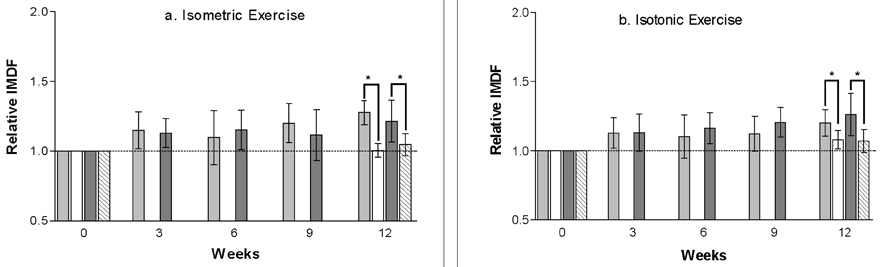
- Strength training is one of the most common exercises practiced in the field of physical therapy or sports training. However, limited methodology is available to evaluate its effect on the target muscle. This study aimed to test the hypothesis that surface electromyographic (EMG) data from both isometric and isotonic exercise can express changes within the muscle during a 12-week strength training program. Ten healthy male volunteer students (5 for training, 5 for controls) from Yonsei University were recruited for evaluation in this study. DeLorme's axiom was practiced for 12 weeks in the dominant elbow flexors and knee extensors of the training group. Tension for 1 repetition maximum and maximal voluntary isometric contraction, and surface EMG information such as the integrated EMG and three variables from the regression line of median frequency (MDF) data were measured at weeks 0, 3, 6, 9, and 12. The limb circumference was measured at weeks 0 and 12. During the strength training, which was enough for the increment of muscle strength and limb circumference, the rectified-integrated EMG and initial MDF increased with a significant linear pattern in both types of contraction. The two surface EMG variables were able to monitor the physiologic muscle changes during the training. Based on these results, we propose that these two surface EMG variables can be used for monitoring electrophysiological changes in the specific muscle that is undergoing the training program, under conditions where the contraction mode for EMG data collection is either static or dynamic.
Keyword
MeSH Terms
Figure
Reference
-
1. Frontera WR, Dawson DM, Slovik DM. Exercise in rehabilitation medicine. 1999. Champaingn, IL: Human Kinetics;41–83.2. Kraemer WJ, Fleck SJ, Evans WJ. Strength and power training: physiological mechanisms of adaptation. Exerc Sport Sci Rev. 1996. 24:363–397.3. Spijkerman DC, Snijders CJ, Stijnen T, Lankhorst GJ. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand J Rehabil Med. 1991. 23:203–206.4. Hakkinen K, Hakkinen A. Neuromuscular adaptations during intensive strength training in middle-aged and elderly males and females. Electromyogr Clin Neurophysiol. 1995. 35:137–147.5. Hakkinen K, Komi PV. Electromyographic changes during strength training and detraining. Med Sci Sports Exerc. 1983. 15:455–460.6. Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979. 58:115–130.7. Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988. 20(5):Suppl. S135–S145.8. Hakkinen K. Neuromuscular adaptation during strength training, aging, detraining, and immobilization. Crit Rev Phys Rehabil Med. 1994. 6:161–198.9. Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc. 1988. 20(5):Suppl. S132–S134.10. Staron RS, Malicky ES, Leonardi MJ, Falkel JE, Hagerman FC, Dudley GA. Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur J Appl Physiol. 1990. 60:71–79.11. Larsson L, Edstrom L, Lindegren B, Gorza L, Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991. 261:93–101.12. Kadi F, Thornell LE. Training affects myosin heavy chain phenotype in the trapezius muscle of women. Histochem Cell Biol. 1999. 112:73–78.13. Andreassen S, Arendt-Nielsen L. Muscle fibre conduction velocity in motor units of the human anterior tibial muscle :a new size principle parameter. J Physiol. 1987. 391:561–571.14. Basmajian JV, De Luca CJ. Muscle alive: their functions revealed by electromyography. 1985. 5th ed. Baltimore: Williams & Wilkins;201–222.15. Macaluso A, De Vito G, Felici F, Nimmo MA. Electromyogram changes during sustained contraction after resistance training in women in their 3rd and 8th decades. Eur J Appl Physiol. 2000. 82:418–424.16. De Luca CJ. Myoelectrical manifestations of localized muscular fatigue in humans. Crit Rev Biomed Eng. 1984. 11:251–279.17. Westbury JR, Shaughnessy TG. Associations between spectral representation of the surface electromyogram and fiber type distribution and size in human masseter muscle. Electromyogr Clin Neurophysiol. 1987. 27:427–435.18. Sadoyama T, Masuda T, Miyata H, Katsuta S. Fibre conduction velocity and fibre composition in human vastus lateralis. Eur J Appl Physiol Occup Physiol. 1988. 57:767–771.19. Gerdle B, Henriksson-Larsen K, Lorentzon R, Wretling ML. Dependence of the mean power frequency of the electromyogram on muscle force and fibre type. Acta Physiol Scand. 1991. 142:457–465.20. Kupa EJ, Roy SH, Kandarian SC, De Luca CJ. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J Appl Physiol. 1995. 79:23–32.21. Solomonow M, Baten C, Smit J, Baratta R, Hermens H, D'Ambrosia R, et al. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J Appl Physiol. 1990. 68:1177–1185.22. Biedermann HJ, Shanks GL, Forrest WJ, Inglis J. Power spectrum analyses of electromyographic activity. Discriminators in the differential assessment of patients with chronic low-back pain. Spine. 1991. 16:1179–1184.23. Gerdle B, Elert J. The temporal occurrence of the mean power frequency shift of the electromyogram during maximum prolonged dynamic and static working cycles. Int J Sports Med. 1994. 15:Suppl 1. S32–S37.24. Merletti R, Roy S. Myoelectric and mechanical manifestations of muscle fatigue in voluntary contractions. J Orthop Sports Phys Ther. 1996. 24:342–353.25. Gerdle B, Larsson B, Karlsson S. Criterion validation of surface EMG variables as fatigue indicators using peak torque: a study of repetitive maximum isokinetic knee extensions. J Electromyogr Kinesiol. 2000. 10:225–232.26. Potvin JR. Effects of muscle kinematics on surface EMG amplitude and frequency during fatiguing dynamic contractions. J Appl Physiol. 1997. 82:144–151.27. Gerdle B, Karlsson S, Crenshaw AG, Elert J, Friden J. The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. Eur J Appl Physiol. 2000. 81:2–10.28. Moritani T, Gaffney FD, Carmichael T, Hargis J. Interrelationships among muscle fiber types, electromyogram, and blood pressure during fatiguing isometric contraction. 1985. Champaingn, IL: Human Kinetics;20–26.29. Eberstein A, Beattie B. Simultaneous measurement of muscle conduction velocity and EMG power spectrum changes during fatigue. Muscle Nerve. 1985. 8:768–773.30. Clancy EA, Bouchard S, Rancourt D. Estimation and application of EMG amplitude during dynamic contractions. IEEE Eng Med Biol Mag. 2001. 20:47–54.31. Masuda K, Masuda T, Sadoyama T, Inaki M, Katsuta S. Changes in surface EMG parameters during static and dynamic fatiguing contractions. J Electromyogr Kinesiol. 1999. 9:39–46.32. Ament W, Bonga GJ, Hof AL, Verkerke GJ. Electromyogram median power frequency in dynamic exercise at medium exercise intensities. Eur J Appl Physiol Occup Physiol. 1996. 74:180–186.33. Edwards RH, Hyde S. Methods of measuring muscle strength and fatigue. Physiotherapy. 1977. 63:51–55.34. Merletti R, Biey D, Biey M, Prato G, Orusa A. On-line monitoring of the median frequency of the surface EMG power spectrum. IEEE Trans Biomed Eng. 1985. 32:1–7.35. Cho SH, Ok JY. Repeatability of the regression lines for filtered median frequency data of surface EMG signals in fatiguing isotonic exercise. Proceedings of the 1st World Congress of ISPRM. 2001. 2001 July 7-13; Amsterdam, Netherlands.36. Kim YM, Cho SH, Lee YH. Characteristics of the regression lines for EMG median frequency data based on the period of regression analysis during fatiguing isotonic exercise. J Korean Acad Univ Train Phys Ther. 2001. 8:63–76.37. Won JI, Cho SH, Yi CH, Kwon OY, Lee YH, Park JM. Characteristics of the fatigue index in EMG power spectrum analysis during isokinetic exercise. J Korean Acad Univ Train Phys Ther. 2001. 8:11–26.38. LeSuer DA, McComick JH, Mayhew JL, Wasserstein RL, Arnold MD. The accuracy of prediction equations for estimating 1-RM performance in the bench press, squat, and deadlift. J Strength Cond Res. 1997. 11:211–213.39. Rainoldi A, Galardi G, Maderna L, Comi G, Lo Conte L, Merletti R. Repeatability of surface EMG variables during voluntary isometric contractions of the biceps brachii muscle. J Electromyogr Kinesiol. 1999. 9:105–119.40. Mannion AF, Dolan P. Relationship between myoelectric and mechanical manifestations of fatigue in the quadriceps femoris muscle group. Eur J Appl Physiol Occup Physiol. 1996. 74:411–419.41. Roy SH, De Luca CJ, Casavant DA. Lumbar muscle fatigue and chronic lower back pain. Spine. 1989. 14:992–1001.42. Johnson GW. Labview graphical programming: practical appliance in instrumentation and control. 1997. New York: McGraw-Hill;24–29.43. Merletti R, Fiorito A, Lo Conte LR, Cisari C. Repeatability of electrically evoked EMG signals in the human vastus medialis muscle. Muscle Nerve. 1998. 21:184–193.44. Merletti R, Conte LRL, Sathyan D. Repeatability of electrically evoked myoelectric signals in the human tibialis anterior muscle. J Electromyogr Kinesiol. 1995. 5:67–80.45. Bernardi M, Solomonow M, Baratta RV. Motor unit recruitment strategy of antagonist muscle pair during linearly increasing contraction. Electromyogr Clin Neurophysiol. 1997. 37:3–12.46. Bilodeau M, Cincera M, Gervais S, Arsenault AB, Gravel D, Lepage Y, et al. Changes in the electromyographic spectrum power distribution caused by a progressive increase in the force level. Eur J Appl Physiol Occup Physiol. 1995. 71:113–123.47. Doud JR, Walsh JM. Muscle fatigue and muscle length interaction: effect on the EMG frequency components. Electromyogr Clin Neurophysiol. 1995. 35:331–339.48. Kankaanpaa M, Taimela S, Webber CL Jr, Airaksinen O, Hanninen O. Lumbar paraspinal muscle fatigability in repetitive isoinertial loading: EMG spectral indices, Borg scale and endurance time. Eur J Appl Physiol Occup Physiol. 1997. 76:236–242.49. Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion--comparison of three methods. Phys Ther. 1991. 71:98–104.50. Muller EA. Influence of training and of inactivity on muscle strength. Arch Phys Med Rehabil. 1970. 51:449–462.51. Montes Molina R, Tabernero Galan A, Martin Garcia MS. Spectral electromyographic changes during a muscular strengthening training based on electrical stimulation. Electromyogr Clin Neurophysiol. 1997. 37:287–295.52. Portero P, Bigard AX, Gamet D, Flageat JR, Guezennec CY. Effects of resistance training in humans on neck muscle performance, and electromyogram power spectrum changes. Eur J Appl Physiol. 2001. 84:540–546.53. Kisner C, Colby LA. Therapeutic exercise: foundations and techniques. 2002. 4th ed. Philadelphia: FA Davis;58–141.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects on EMG Level by EMG Biofeedback with Progressive Muscle Relaxation Training on Tension Headache
- The Effect of Forward and Backward Treadmill walking Training on Muscular Strength of Lower Extremities before and after Exhausting Exercise
- Cross Training Effect Following Unilateral Leg Strengthening Exercise
- Effects of high-intensity interval training and strength training on levels of testosterone and physical activity among women with polycystic ovary syndrome
- Longer prolapsed rectum length increases recurrence risk after Delorme’s procedure