Yonsei Med J.
2006 Feb;47(1):1-14. 10.3349/ymj.2006.47.1.1.
Human Papillomavirus Type 16 E5 Protein as a Therapeutic Target
- Affiliations
-
- 1Department of Genetic Engineering, Faculty of Life Science and Technology, Sungkyunkwan University, Suwon, Korea. jsyang@skku.edu
- KMID: 1715868
- DOI: http://doi.org/10.3349/ymj.2006.47.1.1
Abstract
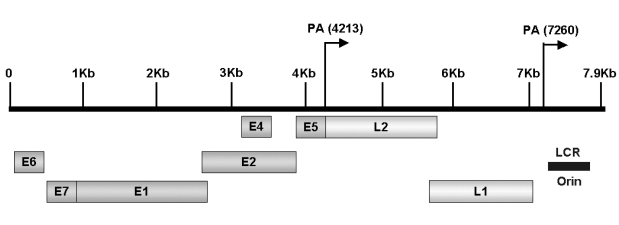
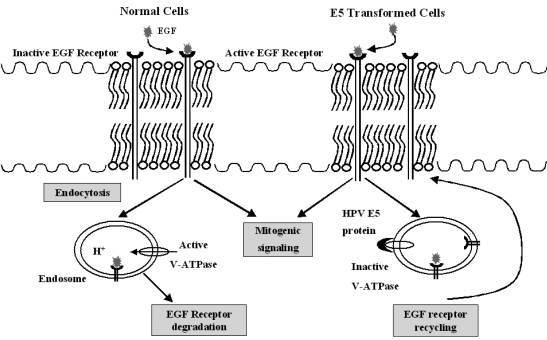
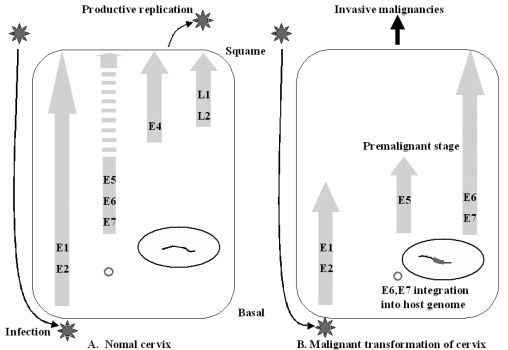
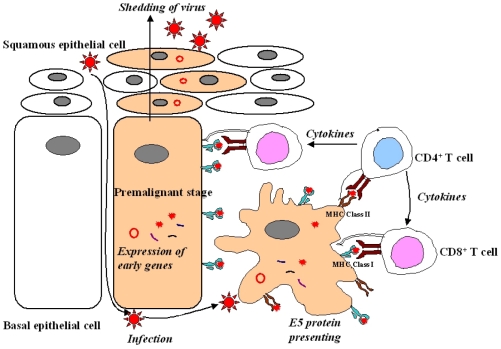
- Cervical cancer is a progressive disease with an onset of one to two decades on average. During the productive replication stage, the Human papillomavirus (HPV) genome is maintained episomally in the infected cervical epithelium and early gene products, including E5, are expressed. Therefore, E5 has a potential to contribute to the HPV-associated carcinogenic process. In invasive malignancies, the HPV genomes are commonly integrated into the host genome, and E6 and E7 genes remain intact. However, the E5 is lost or, if present, under-expressed as compared with the E6 and E7 proteins. This suggests that E5 may play a critical role in the genesis of cervical cancer but less of a role in its persistence or progression. In the initiation of neoplasia and the premalignant stage, there are fewer malignant cells than in the invasive malignancies. Moreover, cells in the invasive malignant stage are found to have a very low level of MHC class I and II, which could hamper the presentation of the antigen and lead to a decreased immune response. Since the E5 protein is likely to play a role during the early tumorigenesis stage, a therapeutic vaccine to target and eliminate the E5-expressing cells may be a good strategy to prevent premalignant lesions from progressing toward invasive cervical cancers. This paper provides an overview of HPV-induced cervical carcinogenesis and strategies for designing prophylactic and therapeutic vaccines to prevent and cure the cervical cancer. In particular, focus will be on the rationale of targeting the E5 protein to develop therapeutic vaccines.
Figure
Cited by 2 articles
-
The Prevalence of Human Papillomavirus Infection in Korean Non-Small Cell Lung Cancer Patients
Moo Suk Park, Yoon Soo Chang, Ju Hye Shin, Dae Joon Kim, Kyung Young Chung, Dong Hwan Shin, Jin Wook Moon, Shin Myung Kang, Chang Hoon Hahn, Young Sam Kim, Joon Chang, Sung Kyu Kim, Se Kyu Kim
Yonsei Med J. 2007;48(1):69-77. doi: 10.3349/ymj.2007.48.1.69.Retraction: Human Papillomavirus Type 16 E5 Protein as a Therapeutic Target. Yonsei Med J 2006;47(1):1-14
Sang-Woo Kim, Joo-Sung Yang
Yonsei Med J. 2011;52(3):551-551. doi: 10.3349/ymj.2011.52.3.551.
Reference
-
1. Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med. 2004; 55:319–331. PMID: 14746524.
Article2. McMurray HR, Nguyen D, Westbrook TF, McAnce DJ. Biology of human papillomaviruses. Int J Exp Pathol. 2001; 82:15–33. PMID: 11422538.
Article3. Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004; 68:362–372. PMID: 15187189.
Article4. Struyk L, van der Meijden E, Minnaar R, Fontaine V, Meijer I, ter Schegget J. Transcriptional regulation of human papillomavirus type 16 LCR by different C/EBPbeta isoforms. Mol Carcinog. 2000; 28:42–50. PMID: 10820487.5. McBride AA, Romanczuk H, Howley PM. The papillomavirus E2 regulatory proteins. J Biol Chem. 1991; 266:18411–18414. PMID: 1655748.
Article6. Heino P, Goldman S, Lagerstedt U, Dillner J. Molecular and serological studies of human papillomavirus among patients with anal epidermoid carcinoma. Int J Cancer. 1993; 53:377–381. PMID: 8381393.
Article7. Chiang CM, Ustav M, Stenlund A, Ho TF, Broker TR, Chow LT. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc Natl Acad Sci USA. 1992; 89:5799–5803. PMID: 1321423.
Article8. Chiang CM, Dong G, Broker TR, Chow LT. Control of human papillomavirus type 11 origin of replication by the E2 family of transcription regulatory proteins. J Virol. 1992; 66:5224–5231. PMID: 1323690.
Article9. Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005; 32(Suppl 1):S7–S15. PMID: 15753007.
Article10. Doorbar J, Ely S, Sterling J, McLean C, Crawford L. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature. 1991; 352:824–827. PMID: 1715519.
Article11. Davy CE, Jackson DJ, Raj K, Peh WL, Southern SA, Das P, et al. Human papillomavirus type 16 E1 E4-induced G2 arrest is associated with cytoplasmic retention of active Cdk1/cyclin B1 complexes. J Virol. 2005; 79:3998–4011. PMID: 15767402.12. DiMaio D, Mattoon D. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene. 2001; 20:7866–7873. PMID: 11753669.
Article13. Genther Williams SM, Disbrow GL, Schlegel R, Lee D, Threadgill DW, et al. Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high-risk human papillomavirus oncogene. Cancer Res. 2005; 65:6534–6542. PMID: 16061632.
Article14. Borzacchiello G, Russo V, Gentile F, Roperto F, Venuti A, Nitsch L, et al. Bovine papillomavirus E5 oncoprotein binds to the activated form of the platelet-derived growth factor beta receptor in naturally occurring bovine urinary bladder tumours. Oncogene. 2005; 25:1251–1260. PMID: 16205631.15. Ishiji T. Molecular mechanism of carcinogenesis by human papillomavirus-16. J Dermatol. 2000; 27:73–86. PMID: 10721654.
Article16. Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989; 8:3905–3910. PMID: 2555178.
Article17. Yasumoto S. HPV16 participates in progressive transformation of normal epidermal cells. Gan To Kagaku Ryoho. 1989; 16:549–561. PMID: 2539784.18. Kessis TD, Slebos RJ, Nelson WG, Kastan MB, Plunkett BS, Han SM, et al. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci USA. 1993; 90:3988–3992. PMID: 8387205.
Article19. Jenison SA, Yu XP, Valentine JM, Galloway DA. Human antibodies react with an epitope of the human papillomavirus type 6b L1 open reading frame which is distinct from the type-common epitope. J Virol. 1989; 63:809–818. PMID: 2463384.
Article20. Tsai TC, Chen SL. The biochemical and biological functions of human papillomavirus type 16 E5 protein. Arch Virol. 2003; 148:1445–1453. PMID: 12898324.
Article21. Oetke C, Auvinen E, Pawlita M, Alonso A. Human papillomavirus type 16 E5 protein localizes to the Golgi apparatus but does not grossly affect cellular glycosylation. Arch Virol. 2000; 145:2183–2191. PMID: 11087100.
Article22. Yang DH, Wildeman AG, Sharom FJ. Overexpression, purification, and structural analysis of the hydrophobic E5 protein from human papillomavirus type 16. Protein Expr Purif. 2003; 30:1–10. PMID: 12821315.
Article23. Auvinen E, Alonso A, Auvinen P. Human papillomavirus type 16 E5 protein colocalizes with the antiapoptotic Bcl-2 protein. Arch Virol. 2004; 149:1745–1759. PMID: 15593417.
Article24. Rodriguez MI, Finbow ME, Alonso A. Binding of human papillomavirus 16 E5 to the 16 kDa subunit c (proteolipid) of the vacuolar H+-ATPase can be dissociated from the E5-mediated epidermal growth factor receptor overactivation. Oncogene. 2000; 19:3727–3732. PMID: 10949926.25. Martin P, Vass WC, Schiller JT, Lowy DR, Velu TJ. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989; 59:21–32. PMID: 2551505.
Article26. Petti L, Nilson LA, DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991; 10:845–855. PMID: 1849073.
Article27. Zhang B, Srirangam A, Potter DA, Roman A. HPV16 E5 protein disrupts the c-Cbl-EGFR interaction and EGFR ubiquitination in human foreskin keratinocytes. Oncogene. 2005; 24:2585–2588. PMID: 15735736.
Article28. Crusius K, Auvinen E, Alonso A. Enhancement of EGF- and PMA-mediated MAP kinase activation in cells expressing the human papillomavirus type 16 E5 protein. Oncogene. 1997; 15:1437–1444. PMID: 9333019.
Article29. Straight SW, Herman B, McCance DJ. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J Virol. 1995; 69:3185–3192. PMID: 7707548.
Article30. Stoppler MC, Straight SW, Tsao G, Schlegel R, McCance DJ. The E5 gene of HPV-16 enhances keratinocyte immortalization by full-length DNA. Virology. 1996; 223:251–254. PMID: 8806560.
Article31. Pim D, Collins M, Banks L. Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene. 1992; 7:27–32. PMID: 1311063.32. Jin H, Wang J, Zuo Y. Expression of c-jun, c-fos and MDM2 mRNA in cultured kerotinocytes through transfecting HPV16. Zhonghua Yi Xue Za Zhi. 2001; 81:171–173. PMID: 11798871.33. Tsao YP, Li LY, Tsai TC, Chen SL. Human papillomavirus type 11 and 16 E5 represses p21(WafI/SdiI/CipI) gene expression in fibroblasts and keratinocytes. J Virol. 1996; 70:7535–7539. PMID: 8892872.
Article34. Hasegawa M, Ohoka I, Yamazaki K, Hanami K, Sugano I, Nagao T, et al. Expression of p21/WAF-1, status of apoptosis and p53 mutation in esophageal squamous cell carcinoma with HPV infection. Pathol Int. 2002; 52:442–450. PMID: 12167102.
Article35. Hernadi Z, Gazdag L, Szoke K, Sapy T, Krasznai ZT, Konya J. Duration of HPV-associated risk for high-grade cervical intraepithelial neoplasia. Eur J Obstet Gynecol Reprod Biol. 2005; 10. 21. [Epub ahead of print].36. Kessis TD, Connolly DC, Hedrick L, Cho KR. Expression of HPV16 E6 or E7 increases integration of foreign DNA. Oncogene. 1996; 13:427–431. PMID: 8710383.37. Wang Q, Griffin H, Southern S, Jackson D, Martin A, McIntosh P, et al. Functional analysis of the human papillomavirus type 16 E1-E4 protein provides a mechanism for in vivo and in vitro keratin filament reorganization. J Virol. 2004; 78:821–833. PMID: 14694114.38. Slebos RJ, Lee MH, Plunkett BS, Kessis TD, Williams BO, Jacks T, et al. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci USA. 1994; 91:5320–5324. PMID: 8202487.
Article39. Wang P, Peng Z, Wang H. Study on the carcinogenic mechanism of human papillomavirus type l6 E7 protein in cervical carcinoma. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2000; 14:117–120. PMID: 11503038.40. Huang FY, Kwok YK, Lau ET, Tang MH, Ng TY, Ngan HY. Genetic abnormalities and HPV status in cervical and vulvar squamous cell carcinomas. Cancer Genet Cytogenet. 2005; 157:42–48. PMID: 15676146.
Article41. Bian J, Connolly DC, Hedrick L, Cho KR. Human papillomavirus type 16 E6 oncogene and expression of P53, RB and PCNA in human cervical carcinoma. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1997; 11:271–273. PMID: 15617346.42. Slebos RJ, Kessis TD, Chen AW, Han SM, Hedrick L, Cho KR. Functional consequences of directed mutations in human papillomavirus E6 proteins: abrogation of p53-mediated cell cycle arrest correlates with p53 binding and degradation in vitro. Virology. 1995; 208:111–120. PMID: 11831691.43. Tamada Y. The biological activities of the human papillomavirus type 16 E6/E7 cDNAs cloned from the SiHa cervical carcinoma cell line. Hokkaido Igaku Zasshi. 1993; 68:849–861. PMID: 8112710.44. Angeline M, Merle E, Moroianu J. The E7 oncoprotein of high-risk human papillomavirus type 16 enters the nucleus via a nonclassical Ran-dependent pathway. Virology. 2003; 317:13–23. PMID: 14675621.
Article45. Pastrana DV, Vass WC, Lowy DR, Schiller JT. HPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology. 2001; 279:361–369. PMID: 11145917.46. Nardelli-Haefliger D, Roden R, Balmelli C, Potts A, Schiller J, De Grandi P. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J Virol. 1999; 73:9609–9613. PMID: 10516071.
Article47. Nardelli-Haefliger D, Lurati F, Wirthner D, Spertini F, Schiller JT, Lowy DR, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005; 23:3634–3641. PMID: 15882523.
Article48. Schiller JT, Lowy DR. Papillomavirus-like particle vaccines. J Natl Cancer Inst Monogr. 2001; 28:50–54. PMID: 11158207.
Article49. Liu HL, Li WS, Lei T, Zheng J, Zhang Z, Yan XF, et al. Expression of human papillomavirus type 16 L1 protein in transgenic tobacco plants. Acta Biochim Biophys Sin (Shanghai). 2005; 37:153–158. PMID: 15756416.
Article50. Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, et al. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003; 95:1128–1137. PMID: 12902442.
Article51. Mahdavi A, Monk BJ. Vaccines against human papillomavirus and cervical cancer: promises and challenges. Oncologist. 2005; 10:528–538. PMID: 16079320.
Article52. Da Silva DM, Pastrana DV, Schiller JT, Kast WM. Effect of preexisting neutralizing antibodies on the anti-tumor immune response induced by chimeric human papillomavirus virus-like particle vaccines. Virology. 2001; 290:350–360. PMID: 11883199.
Article53. Riezebos-Brilman A, Regts J, Freyschmidt EJ, Dontje B, Wilschut J, Daemen T, et al. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005; 12:1410–1414. PMID: 15843807.
Article54. He Z, Wlazlo AP, Kowalczyk DW, Cheng J, Xiang ZO, Giles-Davis W, et al. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology. 2000; 270:146–161. PMID: 10772987.
Article55. Liu DW, Tsao YP, Kung JT, Ding YA, Sytwu HK, Xiao X, et al. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol. 2000; 74:2888–2894. PMID: 10684306.
Article56. Steller MA, Gurski KJ, Murakami M, Daniel RW, Shah KV, Celis E, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res. 1998; 4:2103–2109. PMID: 9748126.57. Lacey CJ, Thompson HS, Monteiro EF, O'Neill T, Davies ML, Holding FP, et al. Phase IIa safety and immunogenicity of a therapeutic vaccine, TA-GW, in persons with genital warts. J Infect Dis. 1999; 179:612–618. PMID: 9952367.
Article58. Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA, et al. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin Exp Immunol. 2000; 121:216–225. PMID: 10931134.59. Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003; 112:109–117. PMID: 12840065.
Article60. Santin AD, Hermonat PL, Ravaggi A, Chiriva-Internati M, Zhan D, Pecorelli S, et al. Induction of human papillomavirus-specific CD4(+) and CD8(+) lymphocytes by E7-pulsed autologous dendritic cells inpatients with human papillomavirus type 16- and 18-positive cervical cancer. J Virol. 1999; 73:5402–5410. PMID: 10364287.61. Gill DK, Bible JM, Biswas C, Kell B, Best JM, Punchard NA, et al. Proliferative T-cell responses to human papillomavirus type 16 E5 are decreased amongst women with high-grade neoplasia. J Gen Virol. 1998; 79:1971–1976. PMID: 9714245.
Article62. Liu DW, Tsao YP, Hsieh CH, Hsieh JT, Kung JT, Chiang CL, et al. Induction of CD8 T cells by vaccination with recombinant adenovirus expressing human papillomavirus type 16 E5 gene reduces tumor growth. J Virol. 2000; 74:9083–9089. PMID: 10982354.
Article63. Chen YF, Lin CW, Tsao YP, Chen SL. Cytotoxic-T lymphocyte human papillomavirus type 16 E5 peptide with CpG-oligodeoxynucleotide can eliminate tumor growth in C57BL/6 mice. J Virol. 2004; 78:1333–1343. PMID: 14722288.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Human Papillomavirus Type 16 E5 Protein as a Therapeutic Target. Yonsei Med J 2006;47(1):1-14
- Synchronous Verrucous Carcinoma and Squamous Cell Carcinoma of Penis by Different Human Papillomavirus Infections
- Human papillomavirus vaccines: current status and perspectives
- Clinical Significance of Expression of p53 Oncoprotein and Human Papillomavirus in Cervical Cancer
- Detection of Human Papilloma Virus Type 16 and 18 in Adenocarcinoma in situ of the Uterine Cervix