J Korean Med Sci.
2010 Aug;25(8):1222-1227. 10.3346/jkms.2010.25.8.1222.
Propofol and Aminophylline Antagonize Each Other During the Mobilization of Intracellular Calcium in Human Umbilical Vein Endothelial Cells
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Kangwon National University Medical School, Chuncheon, Korea. arim14@kangwon.ac.kr
- 2Department of Molecular and Cellular Biochemistry, Kangwon National University Medical School, Chuncheon, Korea.
- 3Department of Neurosurgery, Kangwon National University Medical School, Chuncheon, Korea.
- KMID: 1714047
- DOI: http://doi.org/10.3346/jkms.2010.25.8.1222
Abstract
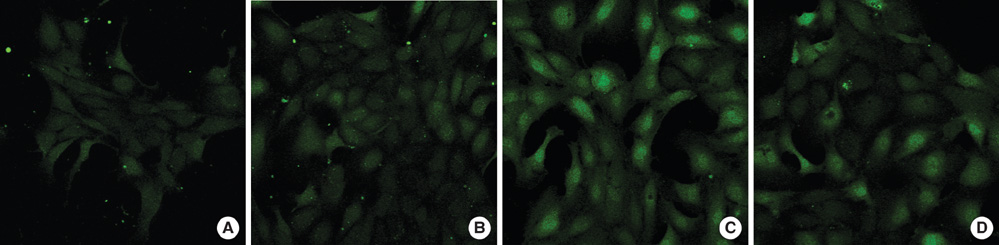
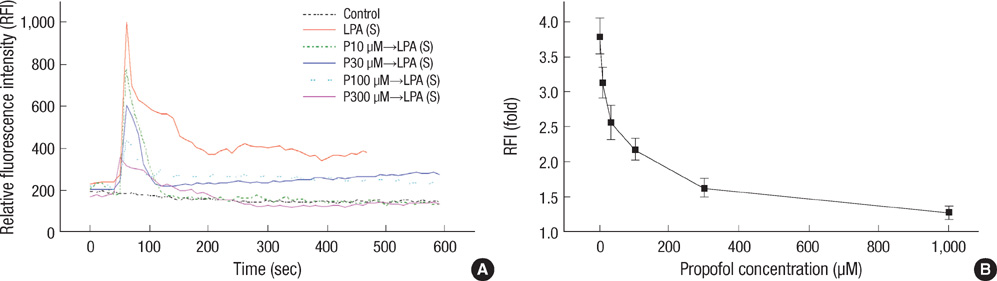
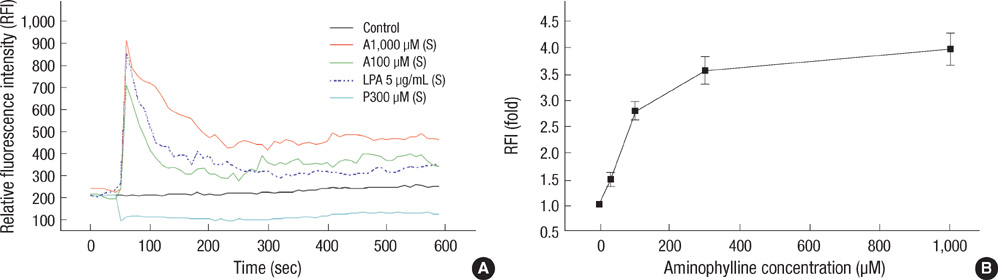
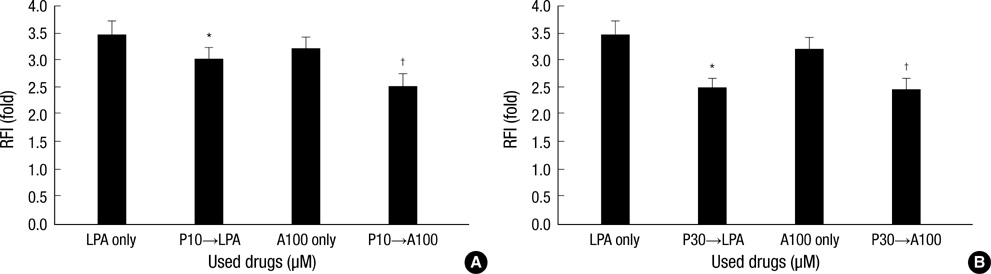
- This study examined whether propofol and aminophylline affect the mobilization of intracellular calcium in human umbilical vein endothelial cells. Intracellular calcium was measured using laser scanning confocal microscopy. Cultured and serum-starved cells on round coverslips were incubated with propofol or aminophylline for 30 min, and then stimulated with lysophosphatidic acid, propofol and aminophylline. The results were expressed as relative fluorescence intensity and fold stimulation. Propofol decreased the concentration of intracellular calcium, whereas aminophylline caused increased mobilization of intracellular calcium in a concentration-dependent manner. Propofol suppressed the lysophosphatidic acid-induced mobilization of intracellular calcium in a concentration-dependent manner. Propofol further prevented the aminophylline-induced increase of intracellular calcium at clinically relevant concentrations. However, aminophylline reversed the inhibitory effect of propofol on the elevation of intracellular calcium by lysophosphatidic acid. Our results suggest that propofol and aminophylline antagonize each other on the mobilization of intracellular calcium in human umbilical vein endothelial cells at clinically relevant concentrations. Serious consideration should be given to how this interaction affects mobilization of intracellular calcium when these two drugs are used together.
Keyword
MeSH Terms
-
Aminophylline/*antagonists & inhibitors/pharmacology
Anesthetics, Intravenous/*antagonists & inhibitors/pharmacology
Bronchodilator Agents/*antagonists & inhibitors/pharmacology
Calcium/*metabolism
Cells, Cultured
Endothelial Cells/*drug effects/metabolism
Endothelium, Vascular/cytology
Humans
Lysophospholipids/pharmacology
Microscopy, Confocal
Propofol/*antagonists & inhibitors/pharmacology
Umbilical Veins/cytology
Figure
Reference
-
1. Cook DJ, Housmans PR. Mechanism of the negative inotropic effect of propofol in isolated ferret ventricular myocardium. Anesthesiology. 1994. 80:859–871.
Article2. Zhou W, Fontenot HJ, Liu S, Kennedy RH. Modulation of cardiac calcium channels by propofol. Anesthesiology. 1997. 86:670–675.
Article3. Barhoumi R, Burghardt RC, Qian Y, Tiffany-Castiglioni E. Effects of propofol on intracellular Ca2+ homeostasis in human astrocytoma cells. Brain Res. 2007. 1145:11–18.4. Ya Deau JT, Morelli CM, Desravines S. Inhibition by propofol of intracellular calcium mobilization in cultured mouse pituitary cells. Anesth Analg. 2003. 97:1325–1330.
Article5. Ryu TG, Kim NS, Min YD, Ha KS, Kong MH, Lim SH. Preventive effects of propofol against the elevation of intracellular Ca2+ and reactive oxygen species induced by lysophosphatidic acid in endothelial cells. Korean J Anesthesiol. 2004. 46:S4–S9.6. Chang HC, Tsai SY, Wu GJ, Lin YH, Chen RM, Chen TL. Effects of propofol on mitochondrial function and intracellular calcium shift in bovine aortic endothelial model. Acta Anaesthesiol Sin. 2001. 39:115–122.7. Lee H, Goetzl EJ, An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am J Physiol Cell Physiol. 2000. 278:C612–C618.8. Panetti TS. Differential effects of sphingosine 1-phosphate and lysophosphatidic acid on endothelial cells. Biochim Biophys Acta. 2002. 1582:190–196.
Article9. Barnes PJ. Drugs for asthma. Br J Pharmacol. 2006. 147:Suppl 1. S297–S303.
Article10. Fanta CH. Asthma. N Engl J Med. 2009. 360:1002–1014.
Article11. Lee ZW, Kweon SM, Kim BC, Leem SH, Shin IC, Kim JH, Ha KS. Phosphatidic acid-induced elevation of intracellular Ca2+ is mediated by RhoA and H2O2 in Rat-2 fibroblasts. J Biol Chem. 1998. 273:12710–12715.12. An S, Bleu T, Zheng Y, Goetzl EJ. Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization. Mol Pharmacol. 1998. 54:881–888.
Article13. Tokumura A, Okuno M, Fukuzawa K, Houchi H, Oka M. Two effects of lysophosphatidic acid on Ca(2+)-movement in cultured bovine adrenal chromaffin cells. J Lipid Mediat Cell Signal. 1996. 14:127–135.
Article14. Coates DP, Monk CR, Prys-Roberts C, Turtle M. Hemodynamic effects of infusions of the emulsion formulation of propofol during nitrous oxide anesthesia in humans. Anesth Analg. 1987. 66:64–70.
Article15. Lückhoff A, Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch Pharmacol. 1990. 342:94–99.
Article16. Roh WS, Ding X, Murray PA. Propofol and thiopental attenuate adenosine triphosphate-sensitive potassium channel relaxation in pulmonary veins. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L636–L643.
Article17. Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989. 341:197–205.
Article18. Xuan YT, Glass PS. Propofol regulation of calcium entry pathways in cultured A10 and rat aortic smooth muscle cells. Br J Pharmacol. 1996. 117:5–12.
Article19. Yang M, Ding X, Murray PA. Differential effects of intravenous anesthetics on capacitative calcium entry in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008. 294:L1007–L1012.
Article20. Wickley PJ, Shiga T, Murray PA, Damron DS. Propofol modulates Na+-Ca2+ exchange activity via activation of protein kinase C in diabetic cardiomyocytes. Anesthesiology. 2007. 106:302–311.21. Stirt JA, Sullivan SF. Aminophylline. Anesth Analg. 1981. 60:587–602.
Article22. Niemand D, Martinell S, Arvidsson S, Ekström-Jodal B, Svedmyr N. Adenosine in the inhibition of diazepam sedation by aminophylline. Acta Anaesthesiol Scand. 1986. 30:493–495.23. Fratacci MD, Shimahara T, Bournaud R, Atlan G. cAMP-dependent modulation of L-type calcium currents in mouse diaphragmatic cells. Respir Physiol. 1996. 104:1–9.
Article24. Mei YA, Le Foll F, Vaudry H, Cazin L. Adenosine inhibits L- and N-type calcium channels in pituitary melanotrophs. Evidence for the involvement of a G protein in calcium channel gating. J Neuroendocrinol. 1996. 8:85–91.
Article25. Ridings JW, Barry SR, Faulkner JA. Aminophylline enhances contractility of frog skeletal muscle: an effect dependent on extracellular calcium. J Appl Physiol. 1989. 67:671–676.
Article26. Mitra A. The current role of intravenous aminophylline in acute paediatric asthma. Minerva Pediatr. 2003. 55:369–375.27. Delbono O, Kotsias BA. Effect of aminophylline-Ca2+ blocker interaction on membrane potential of rat diaphragm fibers. J Appl Physiol. 1993. 74:2745–2749.
Article28. Whitehurst VE, Joseph X, Vick JA, Alleva FR, Zhang J, Balazs T. Reversal of acute theophylline toxicity by calcium channel blockers in dogs and rats. Toxicology. 1996. 110:113–121.
Article29. Ho KM, Ng JY. The use of propofol for medium and long-term sedation in critically ill adult patients: a meta-analysis. Intensive Care Med. 2008. 34:1969–1979.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Propofol attenuates hydrogenperoxide-induced apoptosis in human umbilical vein endothelial cells via multiple signaling pathways
- Preventive Effects of Propofol Aganinst the Elevation of Intracellular Ca2+ and Reactive Oxygen Species Induced by Lysophosphatidic Acid in Endothelial Cells
- Repetition of Apoptosis Induced by Amiloride Derivatives in Human Umbilical Vein Endothelial Cells
- Nitric Oxide-Induced Intracellular Ca2+ Modulation in Macrovascular Endothelial Cells
- In Vitro Culture of Endothelial Cell and Smooth Muscle Cell for Studying Vascular Diseases