J Vet Sci.
2013 Dec;14(4):433-440. 10.4142/jvs.2013.14.4.433.
Pathology of non-thermal irreversible electroporation (N-TIRE)-induced ablation of the canine brain
- Affiliations
-
- 1Department of Small Animal Clinical Sciences, Virginia-Maryland Regional College of Veterinary Medicine, Virginia Tech, Blacksburg, VA 24061, USA. jrossmei@vt.edu
- 2Bioelectromechanical Systems Laboratory, School of Biomedical Engineering and Sciences, Virginia-Tech Wake Forest University School of Biomechanical Engineering, Virginia Tech, Blacksburg, VA 24061, USA.
- 3Department of Biomedical Sciences and Pathobiology, Virginia-Maryland Regional College of Veterinary Medicine, Virginia Tech, Blacksburg, VA 24061, USA.
- 4Wake Forest University School of Medicine, Winston-Salem, NC 27157, USA.
- KMID: 1712310
- DOI: http://doi.org/10.4142/jvs.2013.14.4.433
Abstract
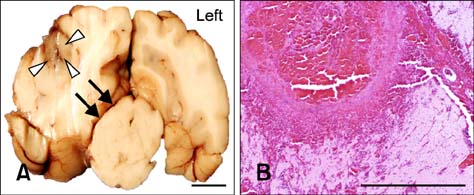
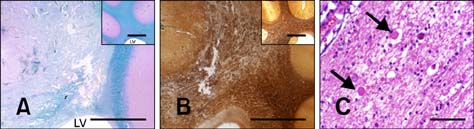
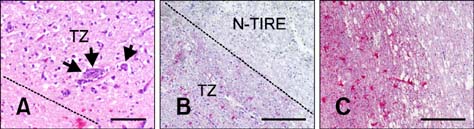
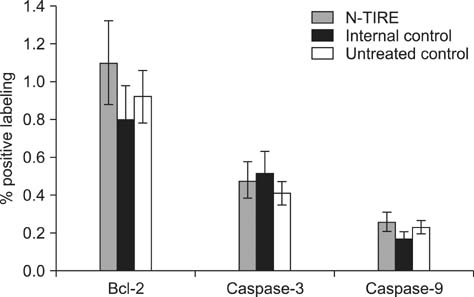
- This study describes the neuropathologic features of normal canine brain ablated with non-thermal irreversible electroporation (N-TIRE). The parietal cerebral cortices of four dogs were treated with N-TIRE using a dose-escalation protocol with an additional dog receiving sham treatment. Animals were allowed to recover following N-TIRE ablation and the effects of treatment were monitored with clinical and magnetic resonance imaging examinations. Brains were subjected to histopathologic and ultrastructural assessment along with Bcl-2, caspase-3, and caspase-9 immunohistochemical staining following sacrifice 72 h post-treatment. Adverse clinical effects of N-TIRE were only observed in the dog treated at the upper energy tier. MRI and neuropathologic examinations indicated that N-TIRE ablation resulted in focal regions of severe cytoarchitectural and blood-brain-barrier disruption. Lesion size correlated to the intensity of the applied electrical field. N-TIRE-induced lesions were characterized by parenchymal necrosis and hemorrhage; however, large blood vessels were preserved. A transition zone containing parenchymal edema, perivascular inflammatory cuffs, and reactive gliosis was interspersed between the necrotic focus and normal neuropil. Apoptotic labeling indices were not different between the N-TIRE-treated and control brains. This study identified N-TIRE pulse parameters that can be used to safely create circumscribed foci of brain necrosis while selectively preserving major vascular structures.
MeSH Terms
Figure
Reference
-
1. Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, Rubinsky B, Mir LM. Tumor ablation with irreversible electroporation. PLoS One. 2007; 2:e1135.
Article2. Atsumi H, Matsumae M, Kaneda M, Muro I, Mamata Y, Komiya T, Tsugu A, Tsugane R. Novel laser system and laser irradiation method reduced the risk of carbonization during laser interstitial thermotherapy: assessed by MR temperature measurement. Lasers Surg Med. 2001; 29:108–117.
Article3. Chen LQ, Wei JS, Lei ZN, Zhang LM, Liu Y, Sun FY. Induction of Bcl-2 and Bax was related to hyperphosphorylation of tau and neuronal death induced by okadaic acid in rat brain. Anat Rec A Discov Mol Cell Evol Biol. 2005; 287:1236–1245.
Article4. Chizmadzhev YA, Zarnitsin VG, Weaver JC, Potts RO. Mechanism of electroinduced ionic species transport through a multilamellar lipid system. Biophys J. 1995; 68:749–765.
Article5. Cosman ER, Nashold BS, Bedenbaugh P. Stereotactic radiofrequency lesion making. Appl Neurophysiol. 1983; 46:160–166.
Article6. Davalos RV, Mir LM, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005; 33:223–231.
Article7. Dev SB, Hofmann GA. Electrochemotherapy-a novel method of cancer treatment. Cancer Treat Rev. 1994; 20:105–115.
Article8. Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006; 53:1409–1415.
Article9. Ellis TL, Garcia PA, Rossmeisl JH Jr, Henao-Guerrero N, Robertson J, Davalos RV. Nonthermal irreversible electroporation for intracranial surgical applications. J Neurosurg. 2011; 114:681–688.
Article10. Esiri M, Squier W, Perl D. Oppenheimer's Diagnostic Neuropathology: A Practical Manual. 3rd ed. Boca Raton: CRC Press;2006. p. 531–537.11. Foster RS, Bihrle R, Sanghvi NT, Fry FJ, Donohue JP. High-intensity focused ultrasound in the treatment of prostatic disease. Eur Urol. 1993; 23:Suppl 1. 29–33.
Article12. Garcia PA, Pancotto TE, Rossmeisl JH Jr, Henao-Guerrero N, Gustafson NR, Daniel GB, Robertson JL, Ellis TL, Davalos RV. Non-thermal irreversible electroporation (N-TIRE) and adjuvant fractionated radiotherapeutic multimodal therapy for intracranial malignant glioma in a canine patient. Technol Cancer Res Treat. 2011; 10:73–83.
Article13. Garcia PA, Rossmeisl JH Jr, Neal RE II, Ellis TL, Olson JD, Henao-Guerreo N, Robertson J, Davalos RV. Intracranial nonthermal irreversible electroporation: in vivo analysis. J Membr Biol. 2010; 236:127–136.14. Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007; 6:287–294.
Article15. Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007; 6:307–312.
Article16. Miklavčič D, Šemrov D, Mekid H, Mir LM. A validated model of in vivo electric field distribution in tissues for electrochemotherapy and for DNA electrotransfer for gene therapy. Biochim Biophys Acta. 2000; 1523:73–83.
Article17. National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington: The National Academies Press;2011. p. 11–124.18. Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007; 6:295–300.
Article19. Rubinsky B. Irreversible electroporation in medicine. Technol Cancer Res Treat. 2007; 6:255–260.
Article20. Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality-clinical implications. Technol Cancer Res Treat. 2007; 6:37–48.
Article21. Shafiee H, Garcia PA, Davalos RV. A preliminary study to delineate irreversible electroporation from thermal damage using the arrhenius equation. J Biomech Eng. 2009; 131:074509.
Article22. Tacke J. Thermal therapies in interventional MR imaging. Cryotherapy. Neuroimaging Clin N Am. 2001; 11:759–765.23. Weaver JC. Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem. 1993; 51:426–435.
Article24. Yakovlev AG, Faden AI. Mechanisms of neural cell death: implications for development of neuroprotective treatment strategies. NeuroRx. 2004; 1:5–16.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Irreversible electroporation of hepatocellular carcinoma: the role of ultrasonography
- Irreversible Electroporation: A Novel Image-Guided Cancer Therapy
- Current Research Status of Irreversible Electroporation for Hollow Viscus Organ of Gastrointestinal Tract
- Irreversible Electroporation of a Hepatocellular Carcinoma Lesion Adjacent to a Transjugular Intrahepatic Portosystemic Shunt Stent Graft
- Irreversible electroporation for the treatment of pancreatic neuroendocrine tumors