J Vet Sci.
2013 Dec;14(4):425-432. 10.4142/jvs.2013.14.4.425.
Morphology and histology of the adult Paramphistomum gracile Fischoeder, 1901
- Affiliations
-
- 1Department of Anatomy, Faculty of Medicine, Srinakharinwirot University, Bangkok 10110, Thailand.
- 2Division of Agricultural Science, Mahidol University, Kanchanaburi Campus, Kanchanaburi 71150, Thailand. panat.anu@mahidol.ac.th
- 3Department of Anatomy, Faculty of Science, Mahidol University, Bangkok 10400, Thailand.
- KMID: 1712309
- DOI: http://doi.org/10.4142/jvs.2013.14.4.425
Abstract
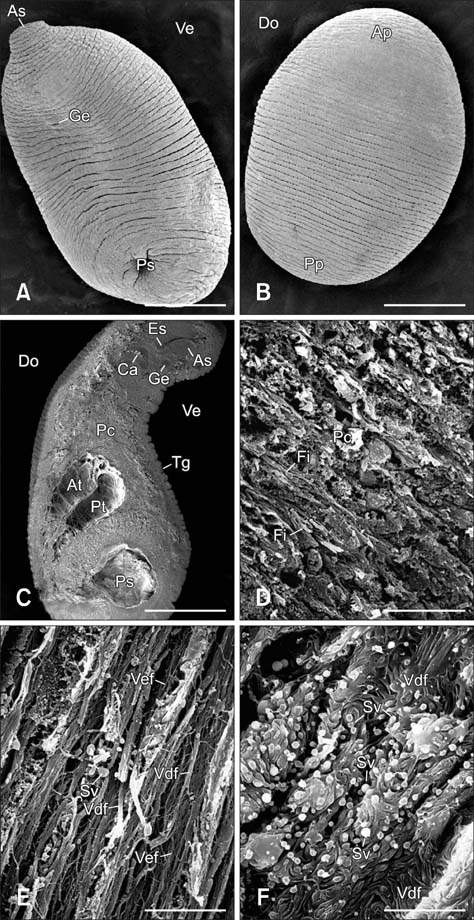
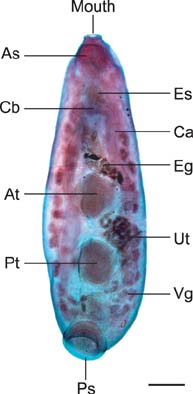
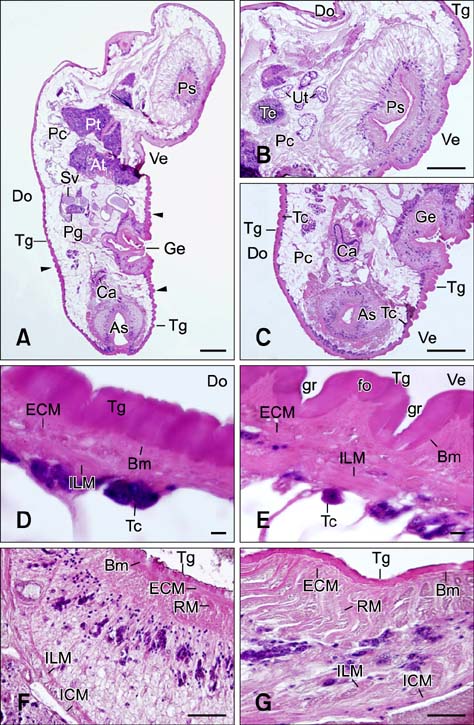
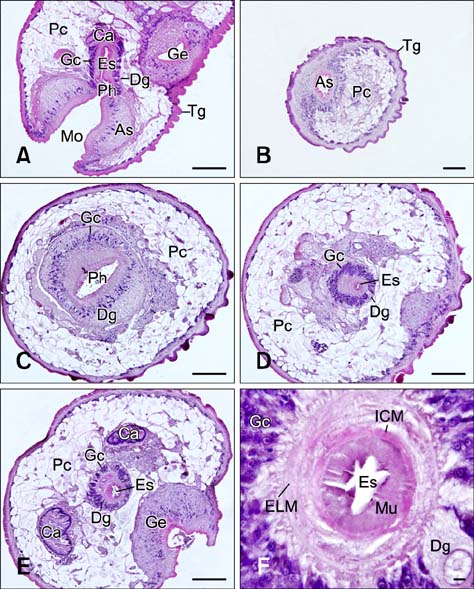
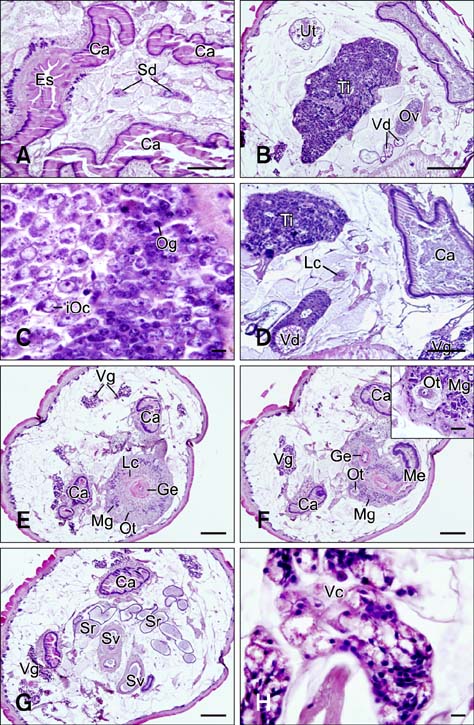
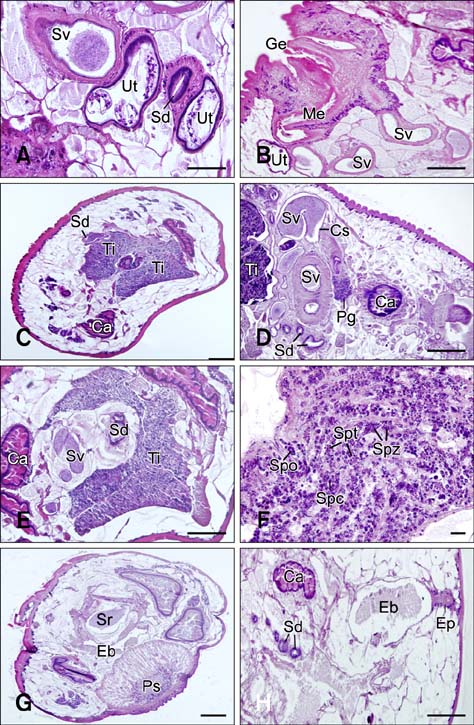
- In the present study, we evaluated the histological morphology of the adult Paramphistomum (P.) gracile. Adult flukes with bodies 5~15 mm in length and 2~7 mm in width were subjected to histological analysis. Longitudinal and transversal serial-sections were stained with hematoxylin and eosin, and examined. The body surface and longitudinal section of P. gracile were also assessed using scanning electron microscopy. In this species, the anterior sucker and posterior sucker (acetabulum) were present on an anterior and posterior part of the body, respectively. The major folds were located in the areas of the anterior sucker, genital canal, and posterior sucker. The fluke membrane was spineless at the tegument surface and in the tegument tissue. Histological data showed structural-systematic characteristics of the digestive tract, reproductive tract, excretory tract, copulatory organs, connective tissues, and muscle tissues. We attempted to elucidate the histological characteristics of P. gracile that might increase the knowledge and understanding of rumen fluke morphology.
Keyword
MeSH Terms
-
Animals
Cattle
Cattle Diseases/*parasitology/pathology
Female
Male
Microscopy, Electron, Scanning/veterinary
Rumen/parasitology
Stomach Diseases/parasitology/pathology/*veterinary
Thailand
Trematoda/*anatomy & histology/isolation & purification/ultrastructure
Trematode Infections/parasitology/pathology/*veterinary
Figure
Reference
-
1. Anuracpreeda P, Chawengkirtikul R, Tinikul Y, Poljaroen J, Chotwiwatthanakun C, Sobhon P. Diagnosis of Fasciola gigantica infection using a monoclonal antibody-based sandwich ELISA for detection of circulating cathepsin B3 protease. Acta Trop. 2013; 127:38–45.
Article2. Anuracpreeda P, Panyarachun B, Ngamniyom A, Tinikul Y, Chotwiwatthanakun C, Poljaroen J, Sobhon P. Fischoederius cobboldi: A scanning electron microscopy investigation of surface morphology of adult rumen fluke. Exp Parasitol. 2012; 130:400–407.
Article3. Anuracpreeda P, Poljaroen J, Chotwiwatthanakun C, Tinikul Y, Sobhon P. Antigenic components, isolation and partial characterization of excretion-secretion fraction of Paramphistomum cervi. Exp Parasitol. 2013; 133:327–333.
Article4. Anuracpreeda P, Wanichanon C, Chaithirayanon K, Preyavichyapugdee N, Sobhon P. Distribution of 28.5 kDa antigen in tegument of adult Fasciola gigantica. Acta Trop. 2006; 100:31–40.5. Anuracpreeda P, Wanichanon C, Sobhon P. Paramphistomum cervi: antigenic profile of adults as recognized by infected cattle sera. Exp Parasitol. 2008; 118:203–207.
Article6. Awad AH, Probert AJ. Scanning and transmission electron microscopy of the female reproductive system of Schistosoma margrebowiei Le Roux 1933. J Helminthol. 1990; 64:181–192.
Article7. Dawes B. A histological study of the caecal epithelium of Fasciola hepatica L. Parasitology. 1962; 52:483–493.
Article8. Gresson RAR. Spermatogenesis in the hermaphroditic digenea (trematoda). Parasitology. 1965; 55:117–125.
Article9. Guk SM, Chai JY, Sohn WM, Kim YM, Sim S, Seo M. Microphallus koreana n. sp. (Trematoda: Microphallidae) transmitted by a marine crab, Macrophthalmus dilatatus. Korean J Parasitol. 2008; 46:165–169.
Article10. Gupta BC, Parshad VR, Guraya SS. Histochemical studies on eggshell formation in Paramphistomum cervi (Digenea: Paramphistomatidae). J Helminthol. 1987; 61:59–64.
Article11. Gupta NK, Nakhasi U. On some amphistomid parasites from India (part I). Rev Iber Parasitol. 1977; 37:205–225.12. Gupta PP, Singh B, Dutt SC. A note on amphistomiasis in an adult buffalo. Indian Vet J. 1978; 55:491–492.13. Hanna REB, Williamson DS, Mattison RG, Nizami WA. Seasonal reproduction in Paramphistomum epiclitum and Gastrothylax crumenifer, rumen paramphistomes of the Indian water buffalo and comparison with the biliary paramphistome Gigantocotyle explanatum. Int J Parasitol. 1988; 18:513–521.
Article14. Horak IG. Paramphistomiasis of domestic ruminants. Adv Parasitol. 1971; 9:33–72.
Article15. Hyman LH. The invertebrates. Vol. 11. New York: McGraw-Hill Book Company;1951. p. 219–239.16. Le TH, Nguyen VD, Phan BU, Blair D, McManus DP. Case report: unusual presentation of Fasciolopsis buski in a Viet Namese child. Trans R Soc Trop Med Hyg. 2004; 98:193–194.
Article17. Meemon K, Khawsuk W, Sriburee S, Meepool A, Sethadavit M, Sansri V, Wanichanon C, Sobhon P. Fasciola gigantica: histology of the digestive tract and the expression of cathepsin L. Exp Parasitol. 2010; 125:371–379.
Article18. Meepool A, Wanichanon C, Viyanant V, Sobhon P. Development and roles of vitelline cells in eggshell formation in Fasciola gigantica. Invertebr Reprod Dev. 2006; 49:9–17.
Article19. do Nascimento CG, do Nascimento AA, Mapeli EB, Tebaldi JH, Duarte JMB, Hoppe EGL. Natural infection by Paramphistomoidea Stiles and Goldberger, 1910 trematodes in wild Marsh Deer (Blastocerus dichotomus Illiger, 1815) from Sérgio Mottas's hydroelectric power station flooding area. Rev Bras Parasitol Vet. 2006; 15:133–137.20. Nikitin VF. Paramphistomiasis of cattle. Veterinariia. 1972; 48:79–81.21. Panyarachun B, Sobhon P, Tinikul Y, Chotwiwatthanakun C, Anupunpisit V, Anuracpreeda P. Paramphistomum cervi: surface topography of the tegument of adult fluke. Exp Parasitol. 2010; 125:95–99.22. Prasitirat P, Chompoochan T, Nithiuthai S, Wongkasemjit S, Punmamoamg T, Pongrut P, Chinone S, Itagaki H. Prevalence of amphistomes of cattle in Thailand. Parasitol Hung. 1997; 29/30:27–32.23. Prasitirat P, Nithiuthai S, Ruengsuk K, Kitwan P, Bunmatid C, Roopan S, Itagaki H. Efficacy of bithionol sulfoxide, niclosamide and fenbendazole against natural rumen fluke infection in cattle. Helminthologia. 1997; 34:155–157.24. Rhee JK, Kim YH, Park BK. The karyotype of Paramphistomum cervi (Zeder, 1790) from Korean cattle. Korean J Parasitol. 1987; 25:154–158.
Article25. Rolfe PF, Boray JC. Chemotherapy of paramphistomosis in cattle. Aust Vet J. 1987; 64:328–332.
Article26. Silva TB, Rossellini M, Dal Pai Silva M, Silva RJ. Histological characterization of Sticholecitha serpentis Prudhoe, 1949 (digena, bieriidae, sticholecithinae), parasite of Bothrops moojeni Hoge, 1966 (serpentes, viperidae). J Venom Anim Toxins Incl Trop Dis. 2005; 11:510–531.27. Terasaki K, Itagaki T, Shibahara T, Noda Y, Moriyama-Gonda N. Comparative study of the reproductive organs of Fasciola groups by optical microscope. J Vet Med Sci. 2001; 63:735–742.
Article28. Wang CR, Qiu JH, Zhu XQ, Han XH, Ni HB, Zhao JP, Zhou QM, Zhang HW, Lun ZR. Survey of helminths in adult sheep in Heilongjiang Province, People's Republic of China. Vet Parasitol. 2006; 140:378–382.
Article29. Willey CH, Godman GC. Gametogenesis, fertilization and cleavage in the trematode, Zygocotyle lunata (paramphistomidae). J Parasitol. 1951; 37:283–296.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Note on Ileodictyon gracile(Clathraceae) in Korea
- The karyotype of Paramphistomum cervi (Zeder, 1790) from Korean cattle
- The karyotype of Paramphistomum explanatum (Creplin, 1849) obtained from Korean cattle
- Immunoelectrophoresis for Fasciola hepatica
- Epizoological survey on infestation rate of helminths in Korean native cattle.